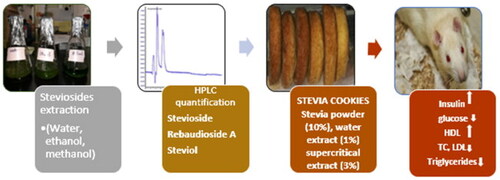
Abstract
Stevia rebaudiana is zero-calorie bio-sweetener and furnishes the best nutrition out of it. The study was conducted to characterize the extracts from water, ethanol, methanol, and supercritical fluid for Steviosides/SGs. The compounds found in appreciable quantities were Stevioside (665.34 ± 27.27 to 1107.95 ± 50.96 mg/kg), Rebaudioside A (383.38 ± 17.25 to 792.15 ± 38.02 mg/kg) and Steviol (357.26 ± 14.64 to 485.25 ± 22.32 mg/kg). Stevia cookies were prepared by replacing sucrose with Stevia powder (10%), water extract (1%), and supercritical extract (3%). Bio-efficacy rats modeling trials were done to check their impact against hyperglycemia and hypercholesterolemia. Blood glucose level was reduced from 4.54 to 7.00%, insulin level was increased by 4.27 to 6.13% due to diets enriched with stevia powder and extracts as compared to the control. In hypercholesterolemic rats, up surged cholesterol level was reported to reduce as a function of Stevia powder and extracts from 1.28 to 5.47%. Substantial increment in HDL, and reduction in LDL and triglycerides was recorded in Stevia leaves powder and extracts treatments by 2.79–6.66%, 2.68–4.16%, and 3.12–5.36, respectively. Stevia cookies administration in rats showed non-significant impact on the liver, renal, and hepatological parameters, while the safety of cookies was improved. Therefore, it can be concluded from the outcomes that in addition to sweetness, Stevia health health-beneficial effects make it suitable to be included in cookies.
Classification:
1. Introduction
Diabetes mellitus, poses a potentially devastating medical condition that has surged in prevalence over recent decades, now standing as a significant public health challenge of the twenty-first century. It is a chronic metabolic disorder and appears when insulin production is either not enough to meet the body’s requirements or the body cells are not responding correctly. Diabetes is also concomitant with many non-communicable diseases (NCDs), such as organ dysfunction or the failure of the blood vessels, heart, and kidneys, thus predisposing to hypertension, renal disease, ocular diseases, stroke, heart failure, obesity, diet disorders (Chowdhury et al., Citation2022). The difficulties of diabetes mellitus include stroke, coronary heart disease, peripheral neuropathy, retinopathy, kidney malfunction, and peripheral vascular disorder (Tomic et al., Citation2022).
Natural foods that are sweet in taste are glucose-rich and tend to elevate the body’s glucose level, leading to enhancement in the production of insulin. However, intense natural sweeteners including Stevia offer a healthier alternative to sucrose and synthetic sweeteners. Studies on the phytochemical profile of Stevia have indicated that stevia exhibits an antioxidant, showcasing a promising profile of phenolic and flavonoids. Stevia demonstrates efficacy in combating inflammation regulating immunomodulatory attributes and possessing antibacterial properties. Additionally, it aids in reducing blood pressure, lowering postprandial glucose levels, and increasing insulin release in the bloodstream (Jan et al., Citation2021).
It has been approved by the FDA and WHO as Generally Safe to use Stevia in food items. Stevia shows fewer side effects than other zero-calorie sweeteners. It contains stevioside which makes stevia 200 to 300 times sweeter than sucrose (Naik & Poyil, Citation2022). Stevia’s (Stevia rebaudiana) leaves are sweet in taste due to the presence of stevioside and rebaudioside. These properties of the plant’s derivatives have spurred research on their biological activities revealing a multitude of benefits to human health, including antidiabetic, anticarcinogenic, antioxidant, hypotensive, antihypertensive, antimicrobial, anti-inflammatory, and antitumor actions. There are many methods for the extraction of bioactive compounds from stevia including conventional and advanced methods (Iwuozor et al., Citation2023). Regrettably, no research has been reported on the extraction of stevia from different solvents like water, ethanol, and methanol and supercritical fluid extraction. Therefore, the aim of the current research was not only on extraction and quantification of stevioside but also on the development of value-added stevia cookies product and it delves into a hot research topic of diabetes mellitus and cholesterolemia issues as hyperglycemia and hypercholesterolemia.
1.1. Procurement of raw material
Stevia rebaudiana leaves and wheat variety Lasani 2011 were procured from Ayub Agricultural Research Institute (AARI), Faisalabad. Chemicals and standards were purchased from RCI Labscan, Ridelheigh, Sigma Aldrich, and Fluka, etc.
1.2. Preparation of raw material
Stevia leaves were cleaned by washing and screening to remove the extraneous material. Leaves were dried in a hot air oven at 30 ± 5 °C for 6 hours followed by conversion of dried raw leaves into powder using high speed mixer. Afterwards, the powder was kept and stored in air tight bags before analysis. Wheat flour was obtained after sieving and milling in Quadrumate Senior mill (Kadam et al., Citation2011).
1.3. Steviosides extraction
Steviosides were extracted by following two methods viz. solvent extraction and the supercritical fluid extraction.
1.3.1. Steviosides extraction by different solvents
Steviosides from Stevia were extracted by using conventional solvent extractions technique. An amount of 5 g of dry Stevia was added in 100 ml of water, methanol and ethanol with subsequent boiling for 30 min. After 2–3 min. the mixture was re-boiled for short time and kept at room temperature for one to two hours. After that, solid material or residues were removed from the boiled solvents. The extract was heated till it reduced to its half volume and then centrifuged at 10 °C, 2500 rpm for 10 minutes for the separation of the aqueous phase. Then the phases were shifted to 25 mL volumetric flasks and filled to the mark. The solutions were filtered through a membrane filter (0.22 μm) to remove any solid residue before further analysis (Németh & Jánosi, Citation2019). The yield of respective samples was recorded and they were stored at 4 °C.
1.3.2. Supercritical fluid extraction of Steviosides
Supercritical Fluid Extraction (SFE) was employed for Steviosides extraction by using a system model No. SFT-150 (Super Critical Technologies, Institute, USA). The volume of the extractor was 100 ml and approximately 30 g Stevia powder was filled in it. The temperature, pressure and ethanol were independent variables, while ethanol was used as co-solvent. The extraction was carried out at different temperature and pressure combinations i.e. 40 °C, 60 °C, and 80 °C temperatures, while the pressures used were 15, 25, and 35 MPa. The above-mentioned temperature and pressure, levels were maintained automatically throughout the extraction process of one hour. The extracted Steviosides were recovered from the separator after complete removal of CO2 gas from the extractor (Ameer et al., Citation2017). However, among different temperature pressure combination, 60 °C with 25 MPa was selected as best for further analysis because of the extraction volume that was higher in this case.
1.3.3. Quantification of Steviosides through HPLC
Bioactive moieties of Stevia were quantify through Perkin Elmer 200 series HPLC equipped with UV detector and C-18 column following the method of Tsotsou and Potiriadi (Citation2022) with few modifications. After derivation, 20 µL was injected in the HPLC system on a reversed-phase C18 column (250 × 4.6 mm ID, 5-µm particle size; Grace, Lokeren, Belgium). The HPLC system consisted of two LC-20AT pumps and auto sampler from Perkin Elmer, USA. The derivative compounds were eluted with mobile phase (70:30 v/v mixture of acetonitrile: water) used at a constant flow rate of 1 ml/min. Detection was done using a wavelength of UV detector at a wavelength of 210 nm at ambient temperature (Tsotsou & Potiriadi, Citation2022).
The chromatographic analysis run time was 15 minutes. Previously it has reported that in gradient elution mode, analysis took more than 15 minutes to achieve the separation followed by equilibration time (5 min) back to initial conditions. However, isocratic elution mode acquires 15 min or less in order to elute and separate the components of interest, without affecting the resolution. Stevioside, Rebaudioside A and Steviol standards with concentration of 10, 100, 500, and 1000pppm were used for calibration. Stevioside standards with different concentrations were run and their elusions were recorded according to their retention times and peak areas. A linear calibration curve was obtained for all the three standards with their high correlation values for Stevioside, Rebaudioside A and Steviol as R2=0.993, R2=0.998, and R2=0.997, respectively.
2.1. Selection of best treatments
On the basis of the highest Steviosides concentration, cookies selected for efficacy trial were prepared with 10% stevia powder, 3% water extract, and 3% supercritical extracts.
2.2. Preparation of cookies
Stevia cookies were prepared by replacing sucrose in the recipe with Stevia powder or extracts according to the method No. 10-50D given in AACC (2000). Flour, sugar, stevia powder/extract, eggs, oil and baking powder were the ingredients in cookies preparation. Cookies were cooled at room temperature and packed in polythene bags prior to further analyses.
2.3. Efficacy trial
In vivo trials were carried out by using Sprague Dawley rats in the model, procured from National Institute of Health (NIH) Islamabad. The study on rats was carried out in Animal Room of National Institute of Food Science and Technology, University of Agriculture, Faisalabad. For one week period basal diet was provided to acclimatize the rats. The environmental conditions like relative humidity (55 ± 5%) and temperature (23 ± 2 °C) were maintained along with 12 hour light dark period throughout the trial. Normal, hyperglycemic and hypercholesterolemic rats were the three groups on which the study was carried out during the efficacy trial. Baseline value was obtained in the start by sacrificing some rats randomly selected from each group. To evaluate the restorative potential, control and selected Stevia diets () were given to all the three groups during eight-week trial: T0: normal sucrose cookies, T1: Stevia powder cookies, T2: Stevia water extract cookies, T3: Stevia supercritical extract cookies. Feed and water intakes along with body weight were measured for the whole experimental model. At the end of the study, overnight fastened rats were sacrificed for testing serum, lipid profile, glucose and insulin level of blood and serum. Serum samples were collected by centrifuging blood samples at 4000 rpm for 6 min. Serum samples were evaluated for numerous biochemical assays via Microlab 300, Merck, Germany. Some rats were slaughtered to get the baseline values at the initiation of trial, however at the end of study (56th) all rats were sacrificed to collect the blood samples for further analysis. Fluctuations in body weights were recorded on weekly basis while feed and water intakes were calculated on daily basis during whole study.
2.3.1. Study I: Normal rats
In this study, rats were divided into four homogeneous groups fed on normal diet along with provision of respective diets.
2.3.2. Study II: Hyperglycemic rats
In study II, hyperglycemia was induced by feeding rats on 40% sucrose diet thereby recording to its effect on serum glucose and insulin.
2.3.3. Study III: Hypercholesterolemic rats
In study III, 1.5% cholesterol was given along with normal diet in order to induce hypercholesterolemia in rats. Customized Stevia diets were given to diseased rats and their effect was recorded accordingly against HDL (High density lipoproteins), LDL (Low density lipoproteins), cholesterol and triglycerides.
Weight gain or loss due to given diets was recorded on weekly basis during the whole study duration to determine any suppressing or incremental effect of Stevia cookies feed on weight variation. Feed intake was calculated on a daily basis by subtracting the left-over diet from the total diet given during the whole study Water was provided in graduated bottles and daily intake was measured by recording the volume of leftover amount of water in bottles.
2.4. Serum glucose and insulin levels
Glucose concentration of all groups under investigation was determined by following the GOD-PAP protocol elaborated by Aini et al. (Citation2022). However, insulin level was evaluated by Enzyme-linked immunosorbent assays (ELISA) and according to the method of Payne et al. (Citation2022).
2.5. Serum lipid profile analysis
Lipid profile parameters of blood serum i-e cholesterol, HDL (high-density lipoproteins), LDL (low-density lipoproteins), and triglycerides were assessed according to the protocols. Cholesterol concentration was determined by following the CHOD-PAP method. HDL and LDL levels were estimated by the Cholesterol Precipitant method (Khanam et al., Citation2022), while triglycerides concentration in sera samples was assessed by the liquid triglycerides (GPO-PAP) protocol defined by Björvang et al. (Citation2022).
2.6. Liver function tests
Liver functioning major variables especially enzymes like ALT (alanine aminotransferase), ALP (alkaline phosphatase), and AST (aspartate aminotransferase) were determined according to their respective kit methods and protocols. ALT and AST concentrations were assessed by the dinitrophenylhydrazene (DNPH) protocol by employing Sigma Kits 58-50 and 59-50, respectively. However, ALP was determined by the Alkaline Phosphates–DGKC method (Park & Yang, Citation2022).
2.7. Renal function tests
Blood samples of Stevia-fed rats were subjected to GLDH and Jaffe commercial kits methods to estimate urea and creatinine levels that will determine the renal functionality of different groups (Saad et al., Citation2022).
2.8. Hematological analysis
Hematological parameters including red and white blood cells (RBC & WBC) and Platelets count were assessed by adopting the protocols of Jeong et al. (Citation2022).
2.9. Statistical analysis
All results were reported as the mean of triplicates ± S.D (Standard deviation of Mean). The data were analyzed using the Statistix 8.1 software, and the statistical significance of the outcomes was evaluated using analysis of variance (ANOVA) and LSD analysis test according to guidelines of Montgomery (Citation2017).
3. Results
3.1. HPLC quantification of Steviosides
In this study, steviosides (SGs) were quantified from different extracts in water, methanol, ethanol and Supercritical fluid of Stevia leaves. The results were calculated by identifying the chromatographic peaks concerning the retention times of known standards and quantified from the peak areas of the known amounts of standards. The data obtained is presented in and it can be observed a substantial effect of solvents on the extraction of the steviosides in different solvents. Stevioside, the prime Steviol glycoside, comprises three glucose molecules attached to an aglycone. The ent-13-hydroxykaur-16-en-18-oic acid is very stable and 250–300 times sweeter than sucrose. Considering the effect of solvents, the maximum concentration of Stevioside was quantified in supercritical fluid extract, followed by ethanol, methanol, and water extracts with their respective values of 1107.9 ± 50.9, 929.8 ± 39.9, 822.1 ± 36.1 and 665.3 ± 27.3 (mg/kg or ppm). Rebaudioside A was found to be maximum in the supercritical extract, while the smallest amount was found in the water extract (). Supercritical fluid extracts had 792.15 ± 38.02 mg/kg of rebaudioside A, methanol had 456.24 ± 21.44 mg/kg, ethanol extract had 410.89 ± 16.84 mg/kg, while the concentration of 393.8 ± 17.25 mg/kg was found in the water extract. Steviol concentration was found to be maximum in the supercritical fluid extract (485.25 ± 22.32 mg/kg), followed by ethanol extract (435.55 ± 18.72 mg/kg), methanol extract (379.36 ± 16.69 mg/kg), and water extract (357.26±.14.64 mg/kg) which presented the lowest value (). The FDA does not allow the incorporation of methanolic or ethanolic extracts into food, despite the fact that they are subjected to high temperatures. “Ethanol may contain residues of methanol, which has been shown to have a highly toxic type of alcohol that can cause serious and sometimes fatal damage if ingested by humans”. That’s why they were not studied for efficacy study and were studied just for HPLC analysis to quantify Steviosides in Stevia extracts ().
Figure 1. HPLC chromatogram of (a) water, (b) ethanolic, (c) methanolic, and (d) supercritical Stevia extract.

Table 1. Mean values for HPLC quantification of Steviosides in Stevia extracts (mg/kg).
3.2. Efficacy study
Efficacy study was done to analyze the health beneficial effects of Stevia powder and extracts against metabolic disorders employing experimental rodents.
3.2.1. Serum profile analysis
Glucose: The data obtained for glucose level is presented in Hyperglycemic and hypercholesterolemic rats glucose level was significantly (P < 0.05) affected by the Stevia diets, while non-significant (P > 0.05) difference was observed in glucose level for the normal rat group. Mean values for glucose in normal rats were 83.58 ± 2.02, 78.79 ± 7.92, 81.89 ± 3.40, and 82.78 ± 4.27 mg/dL for T0, T1, T2, and T3, respectively. In hyperglycemic rats which exhibited significant variation in glucose level among all the diets, T0 (154.40 ± 3.45 mg/dL) had the maximum glucose level, that decreased prominently in T1 (142.29 ± 1.44 mg/dL), T2 (150.10 ± 2.60 mg/dL), and T3 (148.46 ± 2.15 mg/dL), respectively. Hypercholesterolemic rats showed the maximum glucose level of 145.20 ± 4.15 mg/dL inT1 which significantly lessened to 140.20 ± 2.34 mg/dL (T2), followed by 138.40 ± 2.96 mg/dL (T3) and 131.80 ± 2.38 mg/dL (T2), respectively.
Table 2. Effect of stevia cookies on the serum profile.
Insulin: The data for insulin production is presented in and showed that hyperglycemic and hypercholesterolemic rats were significantly affected (P < 0.05) by the diets, while non-significant difference (P > 0.05) was obtained in normal rats. Mean values for insulin production in normal rats were 7.11 ± 0.34, 7.19 ± 0.16, 7.17 ± 0.35, and 7.09 ± 0.26 µU/mL in T0, T1, T2, and T3 groups, respectively. The maximum value for insulin production in the hyperglycemic group was observed in T1 (13.42 ± 1.81µU/mL) that significantly decreased to 12.72 ± 1.96, 10.86 ± 1.04 and 8.76 ± 1.94 µU/mL in T3, T2, and T0 groups, respectively. However, in hypercholesterolemic, T0 provided the lowest insulin level (10.53 ± 3.60µU/mL) that afterwards enhanced in T1, T2, and T3 groups as 14.21 ± 1.99, 11.77 ± 2.65µU/mL, and 12.56 ± 2.76µU/mL, respectively.
Effect of Stevia on cholesterol: The data obtained () established that diets affected non-significantly (P > 0.05) the cholesterol regulation in Normal rat group. However, significant (P < 0.05) variation in cholesterol production was recorded in hyperglycemic and hypercholesterolemic rats groups. Maximum cholesterol in normal rats was found to be 78.24 ± 5.06 mg/dL (T0) and decreased to 77.76 ± 5.00 mg/dL (T2), 76.91 ± 4.03 mg/dL (T3), and 75.01 ± 7.30 mg/dL (T1), respectively. Mean cholesterol values varied for hyperglycemic rats in such a way that T0 (108.16 ± 6.83 mg/dL) was the maximum that lessened to 99.53 ± 8.06, 101.77 ± 8.89 and 104.80 ± 7.35 mg/dL in T1, T3, and T2, respectively. Similarly, in hypercholesterolemic rat group, higher cholesterol value of 110.93 ± 4.32 mg/dL was observed in T0 followed by T2 with 103.59 ± 8.45 mg/dL, T3 with 101.60 ± 6.78 mg/dL, and T1 with 97.61 ± 9.08 mg/dL.
Table 3. Effect of stevia on the lipid profile.
High density lipoprotein (HDL): It is obvious from the data presented in that diets imparted significant differences (P < 0.05) on HDL level in all the three groups. Mean HDL values recorded in normal rats, exhibited the values of 39.85 ± 4.17, 44.65 ± 5.25, 41.46 ± 7.47 and 42.89 ± 5.95 mg/dL for T0, T1, T2, and T3, respectively. However in hyperglycemic rats, the lowest HDL value of 45.05 ± 2.68 mg/dL was found in T0 and increased significantly in T1 (54.74 ± 3.27 mg/dL), T3 (50.73 ± 6.29 mg/dL) and T2 (48.54 ± 2.40 mg/dL). Mean HDL level for T0 group in hypercholesterolemic rat group was 43.91 ± 4.23 mg/dL that increased to 50.57 ± 6.92 mg/dL in T1, 47.78 ± 1.68 mg/dL in T3 and 46.97 ± 3.28 mg/dL in T2, respectively.
Low density lipoprotein (LDL): The data obtained showed non substantial effect (P > 0.05) of diets on LDL in normal and hyperglycemic rats (), while the hypercholesterolemic rats were significantly affected (P < 0.05). Mean values in normal rats for LDL indicated a maximum value of 31.45 ± 4.30 mg/dL in T0 and decreased to 30.82 ± 3.45, 29.48 ± 4.23, and 27.54 ± 4.21 mg/dL in T1, T2, and T3 groups, respectively. In hyperglycemic rats a LDL value of 49.00 ± 3.54 mg/dL was obtained in T0 group and significantly lowered to 42.77 ± 5.89 mg/dL (T1), 47.16 ± 4.50 mg/dL (T2) and 46.06 ± 4.80 mg/dL (T3). In hypercholesterolemic rat group, mean values for T0, T1, T2 and T3 differed i.e. 56.55 ± 4.36, 51.39 ± 6.84, 54.35 ± 5.68, and 53.78 ± 4.33 mg/dL, respectively.
Triglycerides: The data indicated non-significant diet effect (P > 0.05) on triglycerides in normal rats, while a significant effect (P < 0.05) was recorded for hyperglycemic and hypercholesterolemic groups (). Mean values for normal rats depicted a maximum value of 80.20 ± 7.91 mg/dL in T0 that decreased to 75.20 ± 4.86, 78.17 ± 3.81, and 77.42 ± 7.87 mg/dL in T1, T2, and T3 groups, respectively. However, in hyperglycemic rats, the maximum value for triglycerides was 89.0 ± 6.96 mg/dL in T0 that afterwards significantly reduced (P > 0.05) to 81.53 ± 5.41 mg/dL (T1), 86.0 ± 6.28 mg/dL (T2), and 84.4 ± 8.50 mg/dL (T3). Similarly, for hypercholesterolemic rat group, mean triglycerides values for T0, T1, T2, and T3 differed i.e. 97.80 ± 5.40, 92.18 ± 5.85, 95.86 ± 6.30, and 94.72 ± 7.31 mg/dL, respectively.
3.2.2. Liver functions tests
Liver function tests comprised of aspartate transaminase (AST), alanine transaminase (ALT) and alkaline phosphatase (ALP) determinations were carried out for safety reasons.
Aspartate aminotransferase (AST): The results () have depicted that aspartate aminotransferase (AST) level was non-significantly affected (P > 0.05) in normal rats group and hyperglycemic group, while hypercholesterolemic group was significantly affected (P < 0.05) by the diets. Mean AST values for T0, T1, T2, and T3 groups in normal rat group were recorded as 107.42 ± 4.71, 105.48 ± 3.89, 106.12 ± 2.19, and 106.56 ± 2.48 IU/L, respectively. However, in hyperglycemic rat group, means for AST depicted that maximum value in T0 (130.84 ± 3.31 IU/L) as compared to T1 (126.61 ± 5.07 IU/L), T2 (127.69 ± 4.85 IU/L) and T3 (129.23 ± 3.81 IU/L). On the other hand, AST values for hypercholesterolemic rats were significantly varied in such a way that T0 attained maximum level (122.99 ± 5.63 IU/L) which lessened in T1 (116.43 ± 3.23 IU/L), T2 (117.91 ± 5.52 IU/L), and T3 (120.10 ± 5.49 IU/L).
Table 4. Effect of Stevia on the liver functions tests.
Alanine transaminase (ALT): The results presented in depicted that non-significant variation (P > 0.05) was observed in normal rat group for ALT, while in hyperglycemic rat group and hypercholesterolemic rats significant difference (P < 0.05) was found. Hyperglycemic rat group mean ALT results showed significant variation with the maximum value observed in T0 (54.05 ± 4.19 IU/L) that substantially decreased in the following order: T1 (49.56 ± 1.78 IU/L) < T3 (53.15 ± 4.79 IU/L) < T2 (51.28 ± 5.10 IU/L). Similarly, in hypercholesterolemic rat group, the maximum ALT value of 52.07 ± 3.31 IU/L was found in T0 group which afterwards significantly lowered to 47.54 ± 3.08, 49.60 ± 1.80 IU/L, and 50.47 ± 5.11 IU/L in T1, T2, and T3, respectively.
Alkaline phosphatase (ALP): The results for alkaline phosphatase (ALP) showed significant variations (P < 0.05) in all the groups studied (). Maximum ALP mean value in normal rat group was recorded in T0 (141.54 ± 3.49 IU/L) and significantly decreased in T1 (135.17 ± 9.87 IU/L), T2 (138.77 ± 5.03 IU/L), and T3 (140.95 ± 9.53 IU/L). Similarly, in hyperglycemic rats, the highest ALP value recorded was 185.12 ± 2.64 IU/L (T0), followed by 179.09 ± 6.54 IU/L (T2), 177.34 ± 7.83 IU/L (T3), and 171.11 ± 7.5 IU/L (T1), respectively. ALP recorded in the hypercholesterolemic rat group depicted significant variation (P < 0.05) and the minimum value (193.90 ± 6.43 IU/L) was recorded in T1 while the maximum value (202.19 ± 2.54 IU/L) was seen in T1, followed by T2 and T3 which exhibited 197.05 ± 7.39 and 199.72 ± 7.46 IU/L values, respectively.
3.2.3. Renal function tests
In order to check the effect of stevia powder and its extracts on kidney working and structural integrity, renal functioning parameters including urea and creatinine were assessed.
Urea: The results expressed in for urea showed non-significant effect (P > 0.05) if diets in all the three groups (normal, hyperglycemic and hypercholesterolemic rats). Mean values for urea in normal rats group exhibited the maximum value for T0 (23.64 ± 2.32 mg/dL), while the minimum value was shown in T1 (20.77 ± 1.05 mg/dL), followed by T2 and T3 groups with 22.34 ± 0.91 mg/dL and 22.80 ± 1.91 mg/dL, respectively. In a similar manner, in the hyperglycemic rats group, serum urea value ranged from 27.94 ± 2.65 mg/dL (T1) to 30.12 ± 1.80 mg/dL (T0) group, while T2 and T3 had 28.52 ± 3.12 mg/dL and 28.16 ± 3.52 mg/dL respectively with non-significant variation (P > 0.05) among them. However, hypercholesterolemic rat group had the maximum serum urea value registered for T0 and followed by T3, T2, and T1 with their values of 31.10 ± 3.73 mg/dL, 29.09 ± 2.43 mg/dL, 28.79 ± 2.28 mg/dL, and 27.81 ± 3.42 mg/dL, respectively.
Table 5. Effect of stevia on the renal function tests.
Creatinine: Non-significant variation with diets (P > 0.05) were found in creatinine level () among all the groups studied. In normal rats group, T0 depicted the highest creatinine value of 0.67 ± 0.06 mg/dL, while T1, T2, and T3 groups showed a decreasing trend as follows: 0.63 ± 0.04 < 0.65 ± 0.01 < 0.63 ± 0.02 mg/dL, respectively. Similar trend for creatinine was observed for hyperglycemic rat group with T0 presenting 0.96 ± 0.08 mg/dL that slightly varied (P > 0.05) in T1, T2, and T3 as follows: 0.91 ± 0.05, 0.93 ± 0.02 and 0.94 ± 0.09 mg/dL, respectively. In hypercholesterolemic rats group was non-significantly affected by the diets, the maximum creatinine level of 1.02 ± 0.05 being found in T0 followed by 0.95 ± 0.08, 0.97 ± 0.07, and 0.98 ± 0.06 mg/dL in T1, T2, and T3.
3.2.3. Hematological analysis
Red blood cell count (RBCs): The results for RBC () showed that it was non-significantly (P > 0.05) influenced by the diets in all the three groups (normal, hyperglycemic, and hypercholesterolemic rats). In normal rats group, the maximum RBC count was observed in T1 (6.72 ± 0.44cells/Pl), succeeded by T2, T3, and T0 with 6.58 ± 0.43, 6.44 ± 0.57, and 5.87 ± 0.67cells/Pl, respectively. In the same way, non-significant (P > 0.05) increase of RBC count was recorded in hyperglycemic rats and Hypercholesterolemic rats. The maximum value in hyperglycemic rats was found in T1 (7.29 ± 0.85), followed by T2, T3, and T0 having values of 7.16 ± 0.33, 7.13 ± 0.38 and 6.77 ± 0.52cells/Pl, respectively. However, lower RBC content in T0 (7.17 ± 0.64cells/Pl) was found in the hyperglycemic group, and the values increased in the other treatments: T1 (7.75 ± 0.44cells/Pl), T2 (7.45 ± 0.63cells/Pl), and T3 (7.37 ± 0.32cells/Pl), respectively.
Table 6. Effect of Stevia on the hematological profile.
White blood cells count (WBCs): The results presented in for white blood cells (WBC) count exhibited non-significant differences (P > 0.05) in all the three studied groups. In normal rat group, the highest WBC count was found in T0 (6.35 ± 0.93cells/nL), followed by T3 (6.32 ± 0.52cells/nL), T2 (6.29 ± 0.64), and the minimum observed in T1 (6.16 ± 0.84cells/nL), respectively. On one hand, in hyperglycemic rats group, the maximum WBC amount was seen in T0 (13.16 ± 1.01cells/nL) and the minimum was marked in T1 (12.95 ± 1.04 cells/nL). On the other hand, in hypercholesterolemic rat group, the maximum WBC count was found in T0 (15.03 ± 1.13), the minimum in T1 (14.13 ± 1.60), while for T2 and T3 the values were 14.55 ± 1.75 and 14.47 ± 1.18 cells/nL.
Platelets count (PLC): Non-significant variation (P > 0.05) in platelets count of rats in all the three groups are presented in . In normal rat group, the highest plate count was observed in T1 (7.37 ± 0.15), followed by T3 (7.32 ± 0.16), T2 (7.30 ± 0.20), and T0 (7.26 ± 0.45), accordingly. While the maximum platelets count in hyperglycemic rat group was found in T1 as 6.84 ± 0.29, the minimum was observed in T0 (6.77 ± 0.31). Likewise, in hypercholesterolemic rat group, the maximum platelets count was recorded in T1 while the minimum was found in T0 (6.44 ± 0.38 and 6.38 ± 0.24, respectively).
4. Discussion
Nowadays, Steviol glycoside has great potential to be used as a sugar alternative, taste modernizer and sweetening agent in food products. SGs have great potential as multifunctional ingredients for novel applications in food processing. After Stevioside, the second most prevalent Steviol glycoside is Rebaudioside A, which has sweetening potency higher than Stevioside. Rebaudioside-A has a higher sweeter potency because it is 1.6 to 1.8 times sweeter than stevioside (Mathur et al., Citation2017). In the present study, Rebaudioside A was found to be maximum in supercritical extract, while the minimum amount was found in the water extract. However, stevia raw powder of leaves was used in higher quantities so it gave more profound results like in reduction of glucose and increase in insulin and hypercholesterolemia effect. Moreover, the safety test regarding stevia cookies’ effect on the liver, renal, and hematological showed non-significant changes depicting no effects on the functioning of the liver, kidney, and blood. Steviol is almost completely metabolized from all Steviol glycosides by the intestinal microflora in the lower intestinal tract of humans and rodents (Yang et al., Citation2022). The results of recent findings are by Libik-Konieczny et al. (Citation2021) and they have reported that the highest abundant stevia glycoside in S. rebaudiana Bertoni are stevioside and rebaudioside A. The reported variation in concentration was from 5 to 22% for stevioside and 22 to 61.6% for rebaudioside A, (Libik-Konieczny et al., Citation2021). Similar to our study, among the SGs, the highest concentration was found for stevioside, followed byRebaudioside A and Steviol (Libik-Konieczny et al., Citation2021). Likewise, Ko et al. (Citation2021) studied a microwave-assisted green method for enhanced solubilization of water-soluble curcuminoids using Steviol Glycosides and reported that stevioside concentration was 50–200 mg/mL.
Nowadays, the trend is shifting towards natural sweetener-based products. In this study, stevia cookies were prepared and fed to 3 rat groups (normal, hyperglycemic, and hypercholesterolemic rats). Induction of type I diabetes in rats is mostly carried out by a compound known as Streptozotocin. It causes very rapid depletion of β-cells thereby inducing diabetes, which ultimately reduces insulin release. Cells that get resistant to insulin are the most important factor in the inception and progression of type II diabetes. The findings of Abdel-Aal et al. (Citation2021) on the antidiabetic activity in diabetic rats fed with stevia water extract (400 mg/kg/day) showed significantly increased serum insulin levels which demonstrated that stevia could augment the β-cell number of pancreatic islets in diabetic rats which enhance the secretion of insulin from islets of Langerhans. Another research was conducted on phenyl ethanoid Glycoside from Stevia rebaudiana Bertoni to check its effects on insulin secretion in rats and the authors reported the hypoglycemic effect of stevia (He et al., Citation2019). Moreover, Al-Hamdani (Citation2019) investigated the consumption of stevia leaves and reported a significant reduction in glucose, cholesterol, triglyceride, and total fat by feeding 300–400mg/kg of stevia water extract in all treated diabetes-induced groups of mice. However, Zafrilla et al. (Citation2021) have conducted research on overweight subjects to check the effects of stevia, sucralose, and sucrose in citrus–maqui juices and reported that stevia juice showed a significant increase in the IL-10 concentration.
Moreover, Ahmad et al. (Citation2018) have studied the water extract of Stevia rebaudiana Bertoni leaves effects in albino rats. They reported that the stevia extract decreased the body weight gain of rats by lowering the feed intake in hyperlipidemic rats. Furthermore, administration of stevia extract at different levels significantly (P < 0.05) lowered the total cholesterol from 125.22 ± 5.91 to 110.56 ± 5.81 mg/dl, triglyceride level from 102.13 ± 6.89 to 98.62 ± 7.22 mg/dL, Low-density lipoprotein (LDL) from 33.02 ± 4.79 to 22.77 ± 4.36 mg/dl, very low-density lipoprotein (VLDL) from 21.22 ± 5.79 to 19.33 ± 5.95 mg/dL and LDL/HDL ratios (0.83 ± 1.22 to 0.54 ± 1.66 mg/dL) (Ahmad et al., Citation2018). They also found that the HDL level was improved from 39.76 ± 4.34 to 142.02 ± 4.39 mg/dL in hyperlipidemia rats in comparison to the control groups (Ahmad et al., Citation2018).
Hepatic disorders are evaluated by determining the serum enzymes including ALP, AST, and ALT. Liver functioning is hindered and disturbed if the activity of these enzymes is changed, leading to liver damage. Liver inflammation due to diseased hepatic cells leads to extremely high levels of transaminase in bloodstream that ultimately affect the normal functioning of the body. Xiong et al. (Citation2022) have studied stevia extract effects on finishing pigs and reported that the increase in concentration of stevia residual extract from 100 to 800 mg/kg showed a non-significant difference (P > 0.05) for AST (34.42 to 27.53 UL−1), ALT (54.19 to 51.01 UL−1), and creatinine (175.59 to 171.66 μmol L−1), fact that showed the safety level of stevia. Another research was conducted by Nabi et al. (Citation2021) who studied the hypoglycemic effect of stevia-supplemented nectar and reported that the utilization of nectar for 30 days lowered serum enzyme ASAT and ALAT compared to the diabetic control group. Our results depicted that a non-significant effect (P > 0.05) of Stevia powder on white blood cells and red blood cells was found in hyperglycemic and Stevia-treated hyperglycemic groups. Likewise, Ahmad et al. (Citation2020) supported the recent findings and showed that the levels of platelet counts and mean platelet volume decreased in the albino rats who received stevia water extract and also demonstrated that stevia extract has no adverse effect on liver, renal, and hematological profile of albino rats. Overall, a decreasing trend was recorded in body weight, glucose, cholesterol, LDL, and triglycerides, while an increment in HDL, RBCs, and insulin secretion was recorded due to the replacement of sucrose with stevia leaves powder and extracts.
The study characterized the extracts of Stevia leaves and analyzed their stevioside content. Stevia cookies were prepared by replacing sucrose with Stevia powder, water extract, and supercritical extract. The efficacy study showed that the diets enriched with Stevia powder and extracts had positive effects on hyperglycemia and hypercholesterolemia in rats. The consumption of Stevia cookies resulted in reduced blood glucose levels and increased insulin levels compared to the control group. Moreover, the diets containing Stevia showed a decrease in cholesterol levels and an increase in high-density lipoprotein (HDL) levels, indicating potential benefits for cardiovascular health. There were no significant adverse effects on liver, renal, or hepatological parameters, suggesting the safety of Stevia cookies. Overall, these findings suggest that Stevia, with its health-beneficial effects and zero-calorie nature, is a suitable alternative to sucrose and can be included in cookies as a healthier option.
5. Conclusion
This research gives updated evidence on the potential extenuation of metabolic syndrome in a rat model through the consumption of Stevia cookies. The sweetening agent Stevioside in stevia leaves powder was highest in supercritical fluid extract, followed by ethanol, methanol, and water extracts. The efficacy study on hypercholesterolemic and hyperglycemic rats showed the more pronounced effect of stevia cookies on glucose, insulin, and cholesterol levels, while no distinct difference was observed for the normal rat group. It is worth mentioning that among all stevia leaves powder-based cookies has augmented insulin secretion, and reduced blood glucose level, LDL, cholesterol, and triglycerides while HDL contents have been enhanced non-substantially. Moreover, the kidney, liver, and blood profile showed that stevia has no side effects for utilization in food items. Further research is needed to explore the long-term effects and optimal dosage of Stevia in human subjects.
Author contributions
Conceptualization, M.F.J.C. and I.P.; methodology, I.P.; software, S.M.; validation, T.M., and M.Z.K.; formal analysis, M.A.F., and A.L.; investigation, A.KS.T.; resources, I.C.; data curation, W.K.; writing—original draft preparation, M.F.J.C.; writing—review and editing, C.M., M.U-I. and I.C.; visualization, A.A.A., S.A., and F.K.M.; supervision, I.P.; project administration, T.M.; funding acquisition, I.P., A.A.A., S.A., and T.M. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank University of Agriculture Faisalabad and Khwaja Fareed University Engineering and Information Technology, Rahim Yar Khan for scientific support and provide free asses to journals or books. This project was supported by Researchers Supporting Project Number (RSP-2024/7) King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Notes on contributors
Muhammad Farhan Jahangeer Chughtai
Muhammad Farhan Jahangeer Chughtai PhD Food Technology. He has served as Research Scholar at Purdue University, West Lafayette, Indiana, USA. He is working as Additional Director at Institute of Food Science and Technology, khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, Pakistan. He is working on sweeteners and cereals and expertise in Food Analysis. He has number of publications in reputable journals to his credit.
Imran Pasha
Imran Pasha is serving as Director General at National Institute of Food Science and Technology, University of Agriculture, Faisalabad. He did his Post Docs from Australia and USA. He is seasoned and distinguished scientist in areas of Food and Nutrition with research emphasis on Cereals Science, Public Health and Food Safety. He has completed different research projects of significant importance in areas of Food and Nutrition.
Samreen Ahsan
Samreen Ahsan PhD Food Technology. She has served as Research Scholar at Purdue University, West Lafayette, Indiana, USA. He is working as Assistant Professor at Institute of Food Science and Technology, khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, Pakistan. She is working on Food Safety, Food Microbiology and Food value addition. She has number of publications in reputable journals to her credit.
Tariq Mehmood
Tariq Mehmood PhD Food Technology. Dr. Tariq is working as Assistant Professor in Khwaja Fareed University of Engineering and Information Technology. He has expertise in Food Characterization as well as Fruits and Vegetables Processing.
Muhammad Zubair Khalid
Muhammad Zubair Khalid is a young Food Science researcher. He has authored and co-authored 40 papers in various international journals. His preliminary research focuses on Extraction of Phytoconstituents from Agro Wastes. He has preliminary worked on clean label extraction technique. He is currently working on waste management and green extraction technologies
Muhammad Adil Farooq
Muhammad Adil Farooq is working as Assistant professor in the Institute of Food Science and Technology, Khwaja Fareed University of Engineering and Information Technology. He has earned his expertise in field of starch Science and Technology. He has published many articles at international level.
Atif Liaqat
Atif Liaqat is working as Assistant Professor in the Institute of Food Science and Technology at Khwaja Fareed University of Engineering and Information Technology Rahim Yar Khan. He has also worked as research scholar at Michigan State University East Lansing, USA. His research interests include Food Microbiology, Food Safety and Nutrition.
Adnan Khaliq
Adnan Khaliq is an accomplished academician and researcher, currently serving as an Assistant Professor at the Institute of Food Science and Technology Department Khwaja Fareed University of Engineering and Information Technology. Dr. Adnan Also worked as International Scholar at Nelson Hall of Food Science, Purdue University, USA. Dr. Adnan Khaliq has contributed significantly to his academic discipline. He has published significant number of research articles in the field of Food Science and Nutrition focusing mainly on Dairy Technology, functional foods and food safety.
Saira Tanweer
Saira Tanweer PhD Food Technology. She severed as Research Scholar at Michigan State University USA. She is working as assistant Professor in The Islamia University of Bahawalpur. She is working on biokinetic properties of herbs and spices and earns a number of publications from this project.
Mădălina Ungureanu-Iuga
Mădălina Ungureanu-Iuga is a research scientist in food quality control at the “Ştefan cel Mare” University of Suceava and at the Romanian Academy - “Costin C. Kiriţescu” National Institute of Economic Researches (INCE), Mountain Economy Center (CE-MONT), with a Ph.D. in food engineering. His main research topics include food quality control, food processing, by-products valorization, food security, food safety and consumer behavior. She is co-author of more than 55 papers, 13 inventions patents proposals and more than 65 conference presentations which received many national and international awards. Regarding the international experience, she is collaborating with scientists from the Universidad Autonoma de Estado de Mexico and with the Technical University of Moldova.
Ionica Cotovanu
Ionica Cotovanu PhD Food Engineering. Associate assistant, Ionica Coțovanu is an active researcher in the food product engineering field and is a doctor in Food Engineering since 2021 when she sustained her doctoral thesis. Her research activities focus on enhancing the raw materials characteristics by applying different physical treatments, and the valorization of vegetable by-products to improve the nutritional profile and the quality attributes of various foods such as baked goods, pasta, etc. She has published more than 20 scientific papers.
Abdullah Ali Alkahtane
Abdullah Ali Alkahtane has completed his Ph.D. from University of Minnesota, USA. Presently he has been working as an Associate Professor in Department of Zoology and also, he is a General Counselor and Supervisor of the Advisors Unit- Deputy Ministry of Universities Affair- Ministry of Education. He has 18 years’ experience in both teaching and research activities. He has participated in several International, National conference, symposiums, workshops. He has published more than 40 papers in reputed national, international and ISI journals.
Saleh Alfarraj
Saleh Alfarraj has completed his Ph.D. from King Saud University (KSU), Saudi Arabia, Riyadh and got trained in several labs around the world. Presently he has been working as a Professor in Department of Zoology and also, he is a Training and Community Service Center Manager, King Saud University, Riyadh. He has 18 years’ experience in both teaching and research activities. He has participated in several International, National conference, symposiums, workshops. He has published more than 62 papers in reputed national, international and ISI journals and serving as an editorial board member of reputed journal and also has lifetime membership of Protozoa associations in worldwide.
Felix Kwashie Madilo
Felix Kwashie Madilo is a lecturer in the Food Science and Technology Department of Ho Technical University, Volta Region, Ghana. He is a Faculty of Applied Sciences and Technology board member and a member of Faculty Seminar, Workshop and Durbar committee. He also worked as a visiting researcher and a lecturer at the University of Zuĺuland, South Africa. His research interest includes but not limited to Food Microbiology, Food Fermentation, Food Safety and Hygiene, Food Quality and Management, and Food Processing and Preservation.
References
- Abdel-Aal, R. A., Abdel-Rahman, M. S., Al Bayoumi, S., & Ali, L. A. (2021). Effect of stevia aqueous extract on the antidiabetic activity of saxagliptin in diabetic rats. Journal of Ethnopharmacology, 265, 1. https://doi.org/10.1016/j.jep.2020.113188
- Ahmad, U., Ahmad, R. S., Arshad, M. S., Mushtaq, Z., Hussain, S. M., & Hameed, A. (2018). Antihyperlipidemic efficacy of aqueous extract of Stevia rebaudiana Bertoni in albino rats. Lipids in Health and Disease, 17(1), 175. https://doi.org/10.1186/s12944-018-0810-9
- Ahmad, U., Ahmad, R. S., Shahbaz, M., Imran, A., Haseeb, M., Siddique, F., Yasmin, Z., Ashraf, I., Masroor, A., & Basit, A. (2020). Hematological and toxicological effects of aqueous leaf extract of Stevia rebaudiana Bertoni in normal rat modals. Pakistan Journal of Pharmaceutical Sciences, 33(5(Supplementary), 2249–13.
- Aini, N., Sustriawan, B., Wahyuningsih, N., & Mela, E. (2022). Blood Sugar, Haemoglobin and Malondialdehyde Levels in Diabetic White Rats Fed a Diet of Corn Flour Cookies. Foods (Basel, Switzerland), 11(12), 1819. https://doi.org/10.3390/foods11121819
- Al-Hamdani, H. (2019). Effect of stevia leaves consumption on sugar and other blood characters in diabetes-induced mice. Iraqi Journal of Agricultural Sciences, 50(6). https://doi.org/10.36103/ijas.v50i6.856
- Ameer, K., Chun, B.-S., & Kwon, J.-H. (2017). Optimization of supercritical fluid extraction of steviol glycosides and total phenolic content from Stevia rebaudiana (Bertoni) leaves using response surface methodology and artificial neural network modeling. Industrial Crops and Products, 109, 672–685. https://doi.org/10.1016/j.indcrop.2017.09.023
- Björvang, R. D., Hallberg, I., Pikki, A., Berglund, L., Pedrelli, M., Kiviranta, H., Rantakokko, P., Ruokojärvi, P., Lindh, C. H., Olovsson, M., Persson, S., Holte, J., Sjunnesson, Y., & Damdimopoulou, P. (2022). Follicular fluid and blood levels of persistent organic pollutants and reproductive outcomes among women undergoing assisted reproductive technologies. Environmental Research, 208, 112626. https://doi.org/10.1016/j.envres.2021.112626
- Chowdhury, A. I., Rahanur Alam, M., Raihan, M. M., Rahman, T., Islam, S., & Halima, O. (2022). Effect of stevia leaves (Stevia rebaudiana Bertoni) on diabetes: A systematic review and meta‐analysis of preclinical studies. Food Science & Nutrition, 10(9), 2868–2878. https://doi.org/10.1002/fsn3.2904
- He, J., Zhu, N.-L., Kong, J., Peng, P., Li, L.-F., Wei, X.-L., Jiang, Y.-Y., Zhang, Y.-L., Bian, B.-L., She, G.-M., & Shi, R.-B. (2019). A newly discovered phenylethanoid glycoside from Stevia rebaudiana Bertoni affects insulin secretion in rat INS-1 islet β cells. Molecules (Basel, Switzerland), 24(22), 4178. https://doi.org/10.3390/molecules24224178
- Iwuozor, K. O., Emmanuel, S. S., Ahmed, M. O., Idris, A. M., Emenike, E. C., Saliu, O. D., Qudus, A. H., & Adeniyi, A. G. (2023). Technologies for the extraction and post-extraction of Stevia rebaudiana leaves. Chemistry Africa, 1–25. https://doi.org/10.1007/s42250-023-00787-0
- Jan, S. A., Habib, N., Shinwari, Z. K., Ali, M., & Ali, N. (2021). The anti-diabetic activities of natural sweetener plant Stevia: An updated review. SN Applied Sciences, 3(4), 1–6. https://doi.org/10.1007/s42452-021-04519-2
- Jeong, H. R., Lee, H. S., Shim, Y. S., & Hwang, J. S. (2022). Positive associations between body mass index and hematological parameters, including RBCs, WBCs, and platelet counts, in Korean children and adolescents. Children, 9(1), 109. https://doi.org/10.3390/children9010109
- Kadam, D., Goyal, R., & Gupta, M. (2011). Mathematical modeling of convective thin layer drying of basil leaves. Journal of Medicinal Plants Research, 5(19), 4721–4730.
- Khanam, A., Nessa, A., Zannat, M., Nasreen, S., Sultana, R., Tajkia, T., Naznin, R., & Alam, M. (2022). Study on serum total cholesterol in patient with chronic kidney disease. Mymensingh Medical Journal: MMJ, 31(1), 10–14.
- Ko, J.-A., Ryu, Y.-B., Lee, W.-S., Ameer, K., & Kim, Y.-M. (2021). Optimization of microwave-assisted green method for enhanced solubilization of water-soluble curcuminoids prepared using steviol glycosides. Foods (Basel, Switzerland), 10(11), 2803. https://doi.org/10.3390/foods10112803
- Libik-Konieczny, M., Capecka, E., Tuleja, M., & Konieczny, R. (2021). Synthesis and production of steviol glycosides: Recent research trends and perspectives. Applied Microbiology and Biotechnology, 105(10), 3883–3900. https://doi.org/10.1007/s00253-021-11306-x
- Mathur, S., Bulchandan, N., Parihar, S., & Shekhawat, G. S. (2017). Critical review on steviol glycosides: Pharmacological, toxicological and therapeutic aspects of high potency zero caloric sweetener. International Journal of Pharmacology, 13(7), 916–928. https://doi.org/10.3923/ijp.2017.916.928
- Montgomery, D. C. (2017). Design and analysis of experiments. John Wiley & Sons.
- Nabi, I., Megateli, I., Nait Bachir, Y., Djellouli, S., & Hadj‐Ziane‐Zafour, A. (2021). Effect of stevia and pectin supplementation on physicochemical properties, preservation and in‐vivo hypoglycemic potential of orange nectar. Journal of Food Processing and Preservation, 45(2), e15124. https://doi.org/10.1111/jfpp.15124
- Naik, V., & Poyil, T. (2022). Application of stevia (Stevia rebaudiana Bertoni.) in food products. Pharma Innov, 11, 2056–2060.
- Németh, Á., & Jánosi, S. (2019). Extraction of steviol glycosides from dried Stevia rebaudiana by pressurized hot water extraction. Acta Alimentaria, 48(2), 241–252. https://doi.org/10.1556/066.2019.48.2.12
- Park, H. R., & Yang, E. J. (2022). Combined treatment with herbal medicine and drug ameliorates inflammation and metabolic abnormalities in the liver of an amyotrophic lateral sclerosis mouse model. Antioxidants, 11(1), 173. https://doi.org/10.3390/antiox11010173
- Payne, S. C., Ward, G., Fallon, J. B., Hyakumura, T., Prins, J. B., Andrikopoulos, S., MacIsaac, R. J., & Villalobos, J. (2022). Blood glucose modulation and safety of efferent vagus nerve stimulation in a type 2 diabetic rat model. Physiological Reports, 10(8), e15257. https://doi.org/10.14814/phy2.15257
- Saad, E. A., Zahran, F., El-Ablack, F. Z., & Eleneen, A. M. A. (2022). A newly synthesized derivative and a natural parent molecule: Which would be more beneficial as a future antitumor candidate? Docking and in vivo study. Applied Biochemistry and Biotechnology, 194(11), 5386–5402. https://doi.org/10.1007/s12010-022-04037-w
- Tomic, D., Shaw, J. E., & Magliano, D. J. (2022). The burden and risks of emerging complications of diabetes mellitus. Nature Reviews. Endocrinology, 18(9), 525–539. https://doi.org/10.1038/s41574-022-00690-7
- Tsotsou, G. E., & Potiriadi, I. (2022). A UV/Vis spectrophotometric methodology for quality control of stevia-based extracts in the food industry. Food Control. 137, 108932. https://doi.org/10.1016/j.foodcont.2022.108932
- Xiong, Y., Liu, S., Xiao, H., Wu, Q., Chi, L., Zhu, L., Fang, L., Li, Y., Jiang, Z., & Wang, L. (2022). Dietary stevia residue extract supplementation improves the performance and antioxidative capacity of growing–finishing pigs. Journal of the Science of Food and Agriculture, 102(11), 4724–4735. https://doi.org/10.1002/jsfa.11833
- Yang, Y., Xu, M., Wan, Z., & Yang, X. (2022). Novel functional properties and applications of steviol glycosides in foods. Current Opinion in Food Science, 43, 91–98. https://doi.org/10.1016/j.cofs.2021.11.004
- Zafrilla, P., Masoodi, H., Cerdá, B., García-Viguera, C., & Villaño, D. (2021). Biological effects of stevia, sucralose and sucrose in citrus–maqui juices on overweight subjects. Food & Function, 12(18), 8535–8543. https://doi.org/10.1039/d1fo01160j