Abstract
Triglyceride (TG) buildup in the liver is a hallmark of the metabolic disorder of non-alcoholic fatty liver disease (NAFLD). The four pathogenic categories mainly followed by the NAFLD pathway are hepatocellular carcinoma, non-alcoholic steatosis, steatohepatitis, and progressive fibrosis or cirrhosis. The likelihood of developing NAFLD rises with obesity and insulin resistance. Aging, gender, culture, and ethnicity are all associated with NAFLD, which substantially correlates to liver disorders in both kids and adults. It is prevalent throughout all age ranges. Visceral obesity appears to be exacerbated by poor lifestyle choices such as hypercaloric diets, particularly those high in trans-fat or saturated fat and cholesterol, and sugar-sweetened beverages. Decreasing calorie intake, boosting soy protein and whey ingestion, supplementing with monounsaturated fats, omega-3 fatty acids, and fiber, and changing one’s lifestyle are the initial steps in managing this condition. Polyphenolic substances also have both therapeutic and preventative benefits. Activity and exercise that promotes weight loss and lessen liver fat formation are just as important as maintaining a balanced diet. The patients turn into susceptible to liver transplantation owing to improper concern (the end stage of NAFLD). Examining the impact of nutrition on nonalcoholic fatty liver disease (NAFLD) is the goal of this review. Included in this is a result of an unbalanced intake of macronutrients. The pathogenesis of NAFLD involves nutrition. Individuals with NAFLD must be recommended to restrict calorie consumption and set intake limits when following low-fat/carbohydrate diets.
Reviewing Editor:
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a cluster of abnormalities, marked by the help of varying levels of steatosis, inflammation, hepatocellular damage, and fibrosis, and ultimately these are correlated to obesity. The incidence of NAFLD is higher among those people having hypertension, type 2 diabetes, coronary artery disease (CAD), and dyslipidemia. Persons with NAFLD have a diet history of taking excess carbs and lipids in addition to a diet deficient in PUFAS (polyunsaturated fatty acids) and minerals. Even though therapeutic techniques are also critical for the prevention and management of NAFLD, many patients find it tough to keep nutritional therapies due to their eating behavior and lifestyles, as well as the fact that patient motivation for treatment varies. Patient education based on individual nutritional assessments as well as continued treatment is required for successful nutrition therapy (Yasutake et al., Citation2014).
The pathophysiology of NAFLD is demonstrated by the ‘multiple-hit hypothesis’. The preliminary effect is caused by metabolic syndrome and insulin-resistant, because of an increased number of free fatty acids in hepatocytes, which ends up in a reduction of beta-oxidation. The buildup of fatty acids makes the situation worse through the downregulation of beta-oxidation, which leads to steatosis and liver injury. These are the mechanisms that contribute to infection and fibrosis development. Increased fatty acid content boosts the expression of cytochrome peroxidase 2E1 (CYP2E1) and it will increase lipid peroxidation of the hepatocyte membrane by stimulating the production of reactive oxygen species (Cherkaoui-Malki et al., Citation2012). Endotoxin and tumor necrosis factors (TNF) were proven to be deleterious in the development of alcoholic steatohepatitis (Uesugi et al., Citation2002).
NAFLD is a significant public health issue, not just because of the increased morbidity level, but also because of its prevalence in childhood as well as in the adult population (Sullivan, Citation2010). Apparently in the 2011 National Health and Nutrition Examination Survey, NAFLD, obesity, and type 2 diabetes rates have amplified in lockstep since the 1988–1994 survey, demonstrating that NAFLD is linked to obesity and type-2 diabetes. Furthermore, the superiority of NAFLD in kids and youth has been mounting by approximately 10%. Therefore, the genetic aspect has additionally allied with the commencement of pediatric NAFLD, and the prevalence of obesity is also the foremost hazard issue for causing NAFLD. So, the occurrence is higher in obese children.
Previous research on the managing of NAFLD has shown that diets elevated in trans and saturated fats, little in polyunsaturated (primarily omega-3 fatty acids), elevated in extra sugars, and lessened in dietary fiber are generally correlated with the occurrence of NAFLD (Mouzaki & Allard, Citation2012; Romero-Gómez et al., Citation2017). Despite this testimony, individuals do not utilize nutrients or foods in segregation, and it is widely acknowledged that reviewing dietary patterns is important. Dietary patterns, as opposed to nutrients or foods, accurately reflect true dietary exposure and, as a result, offer further suitable understandings of the relationships between diet and health consequences (Hu, Citation2002). For instance, a current systematic review and meta-analysis of observational studies found that a ‘Western’ dietary pattern, marked by extreme consumption of processed and refined foods, high-fat dairy products, and red meat, is related to a 55% upsurge in NAFLD hazard (Hassani Zadeh et al., Citation2020). A ‘prudent’ dietary pattern, defined by elevated ingestions of fruits, vegetables, whole grain cereals, olive oil, and fish, and the Mediterranean diet (characterized by elevated ingesting of vegetables, fruits, olive oil, legumes, unprocessed grains, nuts, and a small amount of red meat, meat-based foods, and desserts, and a reasonable intake of milk and cheese, fish, and alcohol), on the other hand, has been linked with a 22% and 23% decline in NAFLD risk respectively (Hassani Zadeh et al., Citation2020).
These patterns are influenced by food habits and lifestyle alternatives. Overeating and obesity encompass an effect on the enlargement of NAFLD (Yasutake et al., Citation2014). Although there are little management choices for NAFLD, agreement statements imply that way of lifestyle changes and particularly food therapy are the cornerstone of management. In this article, we enlighten information about NAFLD and speak about the view of diet and vitamins in the control of NAFLD (Sullivan, Citation2010).
Method of searching
The search was established employing keywords and topic categories for dietary habits, NAFLD, and its associated liver challenges, and was carried out in PubMed, the Web of Science-Core Collection, Embase, and research articles, from beginning until November 05, 2023. The searches turned up just English-language articles. We manually scanned the listed publications’ reference lists to find further possibly relevant research.
Effect of caloric intake
A study checked out the effects of a four-week hypercaloric weight-reduction plan that doubled the quantity of energy fed on. Effects of calorie consumption and the intrahepatic triglycerides (IHTG) on liver enzymes and IHTG magnetic resonance spectroscopy (MRS) in fit adults had been contrasted to controls that are matched. The surplus energy was provided with the aid of people who consume a minimum of two fast meals in sequence with a day at eating places, and the diet plan with more vegetables and calories as fats than the average man or woman’s weight loss plan. Individuals had NAFLD; even though IHTG levels improved from 1.1 to 2. Furthermore, with the hypercaloric eating regimen, serum alanine transaminase (ALT) concentrations rose from 22 to 32 to most of sixty-nine U/l (Brøns et al., Citation2009).
Another study investigated the effect of high caloric food content on an individual having type–2 diabetes for 7 days while their meals contained high fructose corn syrup. On the opposite hand, 8 human beings were observed, with no family history of type 2 diabetes. They found that no patient is susceptible to NAFLD. In the control group, the high-fructose corn syrup weight-reduction plan improved76% IHTG, at the same time as in the youngsters with diabetes, IHTG levels improved by up to seventy-nine percent (Lê et al., Citation2009; León-Mimila et al., Citation2021).
For overweight and obese individuals with NAFLD, calorie restriction and weight loss have been confirmed to be beneficial treatments. A study revealed the outcomes of lifestyle rehabilitation, counting behavior change, and dietary remedies without or with exercise, on NAFLD. Merely 14 types of research matched the lifestyle remedy intervention’s rigorous standards. In investigations that include repeated liver biopsies, large improvements in liver enzymes, radiologic symptoms of steatosis, insulin resistance, and liver histology were determined. Another research has discovered a link between hypocaloric diets and weight reduction and lessened IHTG content material. Change in IHTG levels has been monitored after having hypocaloric diets in those trials and diminished in IHTG. Lower IHTG has been related to a decrease in hepatic FFA (free fatty acids) uptake (Larson-Meyer et al., Citation2008; Viljanen et al., Citation2009).
Macronutrient
Though usual calorie expenditure may additionally participate in a function in the pathogenesis and management of NALFD, the macronutrient content of the diet may also be significant (Sullivan, Citation2010). Weight loss and prevention from NAFLD may be performed with a selection of diets (both low in carbohydrates and low in lipids). However, there’s proof that altering the levels of micronutrients or macronutrients in the diet can influence high blood pressure, inflammatory degrees, and serum lipids. Animal research and observational studies monitored that a high carbohydrate weight-reduction plan promotes NAFLD-associated liver disorder (Berná & Romero‐Gomez, Citation2020). The distinct impacts of carbohydrate, fat, & protein ingestion on the management of NAFLD could be defined in the following sections.
Carbohydrate
Carbohydrates are divided into types: simple and complex, simple carbohydrates like sucrose and fructose are a primary cause of NAFLD (Yasutake et al., Citation2014). Fructose is significantly metabolized by the liver, and even though it might ground fewer insulin secretion than glucose or sucrose, it can be extra lipogenic (Havel, Citation2005). Humans that are overfed with fructose had been shown to increase triglycerides (Browning et al., Citation2011) and IHTG (Hyde et al., Citation2019). Moreover, current epidemiologic studies (Ouyang et al., Citation2008) in humans have proven an association between excessive fructose corn syrup intake and NAFLD. Animals have been proven to improve IHTG in reaction to a high fructose weight-reduction plan (Kawasaki et al., Citation2009).
In individuals with NASH (Non-Alcoholic Steatohepatitis), the rate of simple and total carbohydrate consumption was found to be better than in persons with simple steatosis. Excessive consumption of simple carbohydrates has been discovered to cause rapid elevations in blood glucose and reactive hypoglycemia, resulting in a feeling of craving, elevated choice, and end hyperphagia (Yasutake et al., Citation2014). In addition, current genome-wide research has observed many variables that contribute to the boom in liver fat, some of which are related to carbohydrate diets and sugar intake (Fan & Cao, Citation2013). This will increase the emergence of enzymes concerned with fatty acid production via activating sterol regulatory element-binding protein-1c (SREBP-1c), a transcription issue.
The foremost goal of nutritional therapy is to find out about each patient’s ingesting habits, especially simple carbohydrates, and to limit Food items that have excessive carbohydrate/glycemic content material. In comparison, because complex carbohydrates, generally whole grains include nutrients, minerals, antioxidants, and dietary fiber, they’ll help to lessen the initiation and increase of NAFLD (Yasutake et al., Citation2014). Fiber, both insoluble and soluble showed an effective impact on the reduction of NAFLD. Studies have proven the reduction of IHTG in animal models concerning the impact of fiber on NAFLD (Lai et al., Citation2005). Studies regarding dietary consideration in humans encompass contradictory outcomes on the correlation between fiber ingestion and NAFLD (Cortez-Pinto et al., Citation2006; Galisteo et al., Citation2008).
Complex Carbohydrates are demonstrated to assist patients with obesity to lose weight, lower intrahepatic triglyceride levels, and enhance metabolic parameters. However, the link between elongated low carbohydrate diet maintenance and systemic insulin resistance in people has to be shown. Furthermore, low glycemic meal Ingredients (GI is 55 or less), often known as slow-release carbs, have a second-meal impact, which boosts the glycemic response to the next meal. Additionally, such food (for example oats), has been proven to boost hunger, decrease calories consumed, and lower plasma glucose and total cholesterol levels. A low-GI eating regimen does not enhance hepatic insulin sensitivity via itself, but it does lower post-prandial hyperinsulinemia while blended with exercising (Hallberg et al., Citation2018).
A meta-analysis demonstrated with the aim of whole grains lessen the risk of type 2 diabetes, heart diseases, serum insulin levels, fasting glucose, and weight that are allied with the pathogenesis of NAFLD. Patients who expended 54% of their energy from carbohydrates compared to individuals who consumed 35% had a. 6.5-fold expanded hazard of hepatic irritation, as pronounced in one research. For 16 weeks, Ryan and colleagues haphazardly allotted patients to one of two hypocaloric diets: 60% carbohydrate/25 percent fat or 40% carbohydrate/45% fats (15% protein in both) with an equivalent energy deficit (750 kcal/d) (Rehackova et al., Citation2017).
Despite the same weight reduction, patients on the lesser carbohydrate diet possessed lesser ALT concentrations than the ones in the excessive carbohydrate/low-fat weight reduction program. This elaborates that a low-carbohydrate, low-calorie eating regimen, regardless of weight reduction, may be fine for human beings with NAFLD. Patients with NAFLD on an energy-restricted (1200–1400 kcal/d) weight loss program have been compared to a patient with NAFLD on a carbohydrate-confined (20 g/d) food plan. Both activities lost the same amount of weight. By magnetic resonance spectroscopy, the weight reduction led to a reduction in hepatic fats; but patients following a reduced carbohydrate weight loss plan confirmed a greater dramatic reduction in hepatic triglyceride degrees (fifty-five percent) compared to the controlled group of 28% (Mardinoglu et al., Citation2018). As a result, a nutritional care plan for volunteers with NAFLD needs to goal to lessen simple carbs amount while increasing complex carbohydrate intake (Yasutake et al., Citation2014).
Fats
It is a fact that the sort of fats is a long way greater vital than the whole amount of fat because important fatty acids participate in a vital position within the human frame and are required by using the human body in a balanced share, on the other hand, the US Food and drug administration has surpassed the no drug therapy for NAFLD patient, and many scientists nowadays are working on the findings of functional food so that the progression and the chances of developing NAFLD can be decreased by using them (Recena Aydos et al., Citation2019).
Lipids not only are a great source of energy, but many fatty acids are precursors of hormones and other signaling molecules in our body that are important for gene regulation. (Recena Aydos et al., Citation2019). The dietary composition of lipids affects the IHTG. As we have discussed earlier IHTG is reduced by the impact of a low carbohydrate and excessive fat diet that results in low calories helps in weight loss and has a positive effect on many biochemical parameters and a high caloric, high-fat diet raises the IHTG concentration (Hollingsworth et al., Citation2006; Kirk et al., Citation2009).
Research has been done on slightly overweight seemingly healthy women. These women were given an iso-caloric diet for 2 weeks but the percentages of carbs and fats were interchanged. During the low-fat diet, the energy came from fat, carbohydrate, and protein in proportions of 16, 61, and 19% respectively, and 56, 31, and 13% during the excessive-fat diet. The whole calorie consumption was comparable. This increased IHTG. It is reduced by 20% on a low-fat diet and increased by 35% on a high-fat diet (Westerbacka et al., Citation2005). Many studies have shown the effect of saturated fat on NAFLD. A High saturated fat diet demonstrates an improvement in IHTG in animals and the same effect is observed in humans (Haufe et al., Citation2011).
Unsaturated fatty acids containing at least one double bond within the trans configuration are mentioned as trans-fatty acids. They are rarely determined in natural ingredients; however, they’re created with the aid of the hydrogenation procedure to create partially hydrogenated oils. Trans-fatty acids have been connected to diabetes and cardiovascular disease. Trans fatty acids are a great deal more dangerous than saturated fatty acids and they may be no longer tolerated by our bodies in any respect. The American Heart Association recommends that adults who would benefit from reducing LDL cholesterol lessen their ingestion of trans fat and limit their expenditure of saturated fat to 5–6% of total calories (Hormoznejad et al., Citation2020).
Trans fats had a very serious impact on the rat’s liver, a vast difference inside the liver of rats has been seen throughout the experiment of 4 weeks. Three groups (each having 10 rats) of Male Wister rats were taken arbitrarily and their dietary foods were taken in three types. One group of rats was subjected to a 25% margarine diet; the second to a 25% Fresh soybean oil diet (FSO) and 3 were subjected to an oxidized soybean oil (OSO) diet. There was increased fat deposition in the liver of rats (OSO Intake) due to a reduction in antioxidant enzyme activity. Margarine’s diet had adverse effects on the hepatic system such as vacuolation of cytoplasm, and onomatology resulting in cell death. It showed that dietary fat had a direct impact on the liver of rats. Fresh soybean oil did not affect the liver, but margarine and oxidized soybean oil damaged it badly in the form of NAFLD (Dhibi et al., Citation2011).
Polyunsaturated fatty acid (PUFA) and monounsaturated fatty acid (MUFA) have magnificent effects on lipid composition. Essential fatty acids like Omega 3 and 6 are confirmed to be helpful within the remedy of NAFLD and most sufferers with NAFLD are suffering from an imbalance of each omega three and omega 6 fatty acids. 2 g of omega-3 fatty acid supplementation one-year decreases IHTG (Yu et al., Citation2017).
Protein
Elevated protein consumption aids in the natural mobilization of liver fat. The excessive protein impact may be influenced by the quantity of methionine and branched-chain amino acids (BCAA) in the meal. For instance, subsequent supplements with 60 g of whey protein/day (320 g for 4 weeks), while on an apparent ad libitum diet, liver fat level substantially lessened by 20%. According to the latest six randomized controlled trials by the German Institute of Human Nutrition (DIFE) in Potsdam, patients with T2DM and NAFLD had significantly lower liver fat content when they consumed a caloric, protein-rich diet (30% protein) than they did when eating normally. Respondents in the research were divided into two protein groups at random; one got protein largely from plants (such as legumes), whereas the other consumed protein mainly from animals (like beef, mutton, and fish). Both treatment groups identified a substantial diminish in liver fat after six weeks, although the animal protein group showed a larger decrease (−48% vs. −36%) (Worm, Citation2020).
Soy protein appears to inhibit hepatic lipogenesis and growth insulin sensitivity in animals. Only one study on human beings indicates that quick-term ingestion of soy protein as part of a small energy eating regimen might also offer an extra gain for weight loss in obese human beings (Cañete and Agüero, Citation2014). The role of nutritional protein, chiefly the category of dietary protein, in the development of weight problems and its metabolic repercussions remains unknown (Fan & Cao, Citation2013). Protein is abundant in the American diet, making malnutrition rare. Consuming too much protein can have a negative consequence on kidney function (Fan & Cao, Citation2013). As a result, there is insufficient information to make a complete deposition about the influence of eating protein on NAFL (Fan & Cao, Citation2013).
Effect of micronutrients and antioxidants on NAFLD
To inhibit the onset of NAFLD, micronutrients like vitamins and minerals are needed in small concentrations. Micronutrients with antioxidant and immune-modulating properties, like carotenoids, copper, zinc, iron, selenium, choline, and vitamins A, D, E, and C. Antioxidants slow the spread of the illness by acting as a resilience factor. As a consequence, essential micronutrients hinder the body from storing fat by lowering oxidative stress in the liver, raising high-density lipoproteins, lowering low-density lipoproteins, and increasing the quantity of total cholesterol or triglycerides. Patients with NAFLD have indeed been discovered to have reduced blood concentrations of zinc, copper, vitamins A, C, D, E, and carotenoids, which results in lowered immunity and antioxidant capability, both of which possess an adverse effects on the disease’s development (Hassan et al., Citation2021).
Choline
It is an essential vitamin that causes fatty liver in humans who eat a low-choline diet. The dietary need for choline is, however, influenced by estrogen and single nucleotide polymorphisms in choline and folate metabolism genes. Only in postmenopausal women with NAFLD is a decrease in choline consumption linked to increased fibrosis. Hepatocellular inflammation and disease development in NAFLD are thought to be linked to oxidative stress. Green tea contains polyphenolic catechins, which have antioxidant, hypolipidemic, thermogenic, and anti-inflammatory properties and might assist to avoid NAFLD (Fan & Cao, Citation2013).
Vitamin A
Retinoic acid, another name for vitamin A, is produced during its production using phospholipids and unsaturated fatty acids (Leaf & Lansdowne, Citation2014). The primary reserve location for vitamin A is the liver, and vitamin A closely regulates adipose tissue, which is the initial association amid vitamin A and NAFLD. Additionally, retinol has subsequently been linked to the PNPLA3 mutation, which affects a gene that is powerfully allied with NAFLD (Mondul et al., Citation2015; Pirazzi et al., Citation2014). The quiescent hepatic stellate cells (qHSC), which are in charge of fibrogenesis, are where the majority of the vitamin A accumulation in the liver occurs. It is well documented that qHSC reduces their vitamin A content once they are triggered to develop profibrogenic HSC. Additionally, in reaction to vitamin A metabolites, hepatocytes effectively metabolize vitamin A and alter glucose and lipid metabolism (Senoo et al., Citation2010).
The significance of vitamin A in the etiology of NAFLD has been underlined by numerous types of research. Retinoic acid had been exposed to appreciably increase hepatic fat metabolism and decrease liver obesity (Amengual et al., Citation2010). Additionally, vitamin A was found to be capable to prevent adipogenesis in cultured adipocytes at the first phases of the differentiation pathway, according to in vitro research (Marchildon et al., Citation2010). For NAFLD patients, vitamin A supplementation is seen as beneficial (Hassan et al., Citation2021).
Vitamin C
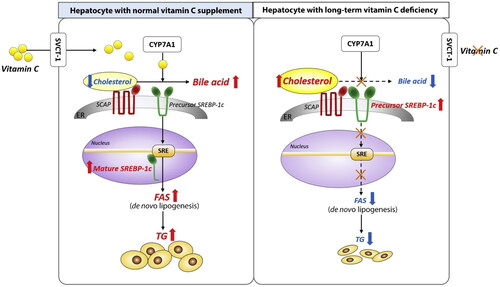
Vitamin C as well recognized as ascorbic acid, is a water-soluble vitamin that should be taken by meals and could not be stored by mammals. Vit. C alleviated histological changes in the liver, hepatic steatosis, and hepatocellular ballooning in a significant way. Some research suggests a moderate link between vitamin C intake and the prevention of NAFLD, particularly in men and non-obese patients. Vit. C has been demonstrated to have a therapeutic impact on NAFLD in another research. Supplementing with ascorbic acid reduced fat deposition, inflammation, and apoptosis in an HFD model of NAFLD, most likely through enhancing the expression of PPARα and genes encoding β-oxidation enzymes (Arroyave-Ospina et al., Citation2021). Vitamin C is also administered in a sufficient quantity for NAFLD patients, according to recommendations, because too much might cause toxicity and thus further disorders (Hassan et al., Citation2021) (). It is clear from that a persistent lack of vitamin C impairs bile acid to cholesterol conversion in the liver.
Figure 1. A persistent lack of vitamin C causes a disturbance in the hepatic de novo lipogenesis pathway by impairing the conversion of bile acid to cholesterol. The current research’ straightforward graphical abstract. Normal hepatocytes exhibit de novo lipogenesis in the left panel. Right panel: Impaired de novo lipogenesis in long-term vitamin C–deficient hepatocytes. CYP7A1: cholesterol 7α-hydroxylase; ER: endoplasmic reticulum; FAS: fatty acid synthase; SCAP: SREBP cleavage-activating protein; SRE: sterol regulatory element; SREBP-1c: sterol regulatory element-binding protein-1c; SVCT-1: sodium-dependent vitamin C transporter 1; TG: triglycerides (Source: Lee et al., Citation2021).
Vitamin D
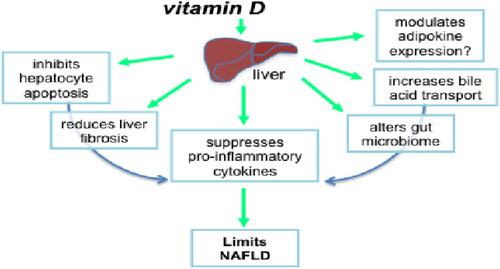
It has a vital position in the inflammatory and autoimmune processes. Insulin resistance, metabolic syndrome, and NAFLD can all be resulting from vitamin D deficiency. Many obese children have an excessive-energy, little vitamin, & mineral dense diet and are not uncovered to enough sunlight. Serum vitamin D concentrations, for example, were poor in 54% of adult Americans. Vitamin D paucity had been related to the harshness of NAFLD interest score and hepatic fibrosis in people, likely due to the improved oxidative stress consequential from vitamin D insufficiency. As a result, earlier than starting up dietary therapy for NAFLD, the position of vitamin D ingestion and serum nutrition D degrees must be decided. Patients with vitamin D insufficiency need to consume vitamin D-wealthy foods at least once a day, along with fish and mushrooms (Yasutake et al., Citation2014) (). According to , vitamin D may slow NAFLD by several interrelated mechanisms.
Figure 2. Through several possible interrelated processes, vitamin D may slow the course of NAFLD (Source: Gorman et al., Citation2015).
Vitamin E
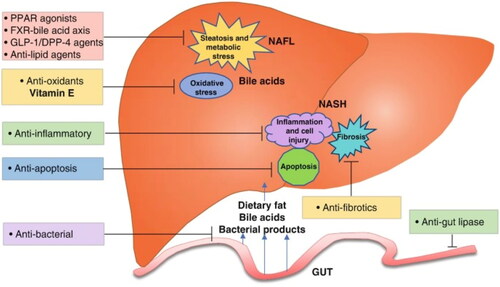
It is a fat-soluble vitamin which is reserved primarily in the liver & fat tissue. Vitamin E was formally recognized as an important component of human health by the FDA in 1959. Vitamin E can be found in a range of foods in nature, such as green leafy vegetables, oils, beef, & eggs. Vitamin E level varies depending on how much vegetable oil is consumed. The Food and Nutrition Board of the National Academy of Sciences in the United States suggests 15 mg of dietary tocopherol/day (Al-Busafi et al., Citation2012) (). The role of Vitamin E in regulating NAFLD can be seen in .
Figure 3. Function of Vitamin E in regulating NAFLD (Source: Banini & Sanyal, Citation2019).
Eight tocopherols make up vitamin E, with a-tocopherol being the most dynamic. Its inclusion in the lipid bilayer of cell membranes stops free radicals from non-enzymatically oxidizing cell components (Hardy et al., Citation2015). However, tocopherols also affect the antioxidant reaction by mounting the synthesis of detoxifying enzymes including SOD, Glutathione Peroxidase, and Catalase. Tocopherols directly scavenge ROS and RNS (Arroyave-Ospina et al., Citation2021). TNF-α, IL-1, and IL-8 expression are all decreased, and lipid peroxidation is inhibited. Relying on the idea that amplified oxidative stress performs a significant responsibility in the pathophysiology of NASH, Lavine’s excellent pilot research exposed the effectiveness of antioxidant treatment with vitamin E for NASH. In this uncontrolled study, 11 pediatric obese individuals with chronically elevated transaminase concentrations for 3 months and echogenic liver texture on ultrasound were treated with vitamin E. (Mehta et al., Citation2002).
Pioglitazone, an insulin sensitizer, and vitamin E together were found to enhance liver histology more than vitamin E singly in pilot testing. Vitamin E improved the ability of ursodeoxycholic acid to normalize ALT in adults with histologically established NAFLD. There had been initiatives to create a powerful antioxidant by combining vitamin C with vitamin E. A four-year randomized experiment assessing atorvastatin plus antioxidants (vitamins E and C) against placebo was just reported by Foster et al., and it revealed a reduction in liver steatosis depending on computed tomography scans. Every study revealed some indication of vitamin E’s usefulness in NASH, though the information was constrained due to limited sample numbers and studies were out in only one place (Al-Busafiet al., Citation2012) (). In , documented studies are shown that vitamin E is beneficial to patients with NAFLD.
Table 1. Displays documented studies on the benefits of vitamin E in people with NAFLD.
Vitamin E’s importance in the management of NASH is supported by its role as a free radical scavenger. Vitamin E is a sequence breaking antioxidant in free radical activities that is a crucial phase in lipid peroxidation & membrane integrity. Besides, the amount of vitamin E utilized in these and succeeding research is relatively high in comparison to the FDA’s recommended daily intake of 15 mg (22.4 IU) of the vitamin (Al-Busafiet al., Citation2012). Current research has shown that it helps non-diabetic people with NASH with their histology. Upon administering vitamin E 800 IU/d for 95 weeks, patients with steatohepatitis significantly outperformed placebo subjects in the large PIVENS investigation (42% vs 19% p < .001) in terms of augmentation. In a trial of childhood NASH, vitamin E was exposed to lessen steatohepatitis in a subset of children with NASH who underwent follow-up liver biopsy (Dyson et al., Citation2015). In a trial of childhood NASH, vitamin E was exposed to lessen steatohepatitis in a subset of children with NASH who underwent follow-up liver biopsy (Dyson et al., Citation2015).
If the transaminases continued to be too high, the therapeutic effects of 400 IU of vitamin E were augmented by 400 IU monthly. Five of the eleven participants had normal liver profiles subsequent to one month, and after two months, four of the other six participants also had healthy liver profiles. After three months of therapy, all participants had transaminases that were normal (Mehta et al., Citation2002). According to recent studies, persons with fibrosis and/or impaired fasting plasma glucose levels appear to benefit from 300 mg/d of vitamin E treatment (Yasutake et al., Citation2014). There have also been reported anti-inflammatory and anti-apoptotic actions that might be connected to cellular injury prevention. Numerous investigations have discovered an inverse relationship between the severity of NAFLD and both Vitamin E serum levels and VitE/Cholesterol ratio-corrected findings (Arroyave-Ospina et al., Citation2021).
There is, though, limited proof that vitamin E enhances supplementary complex histologic features linked to the onset of illness, like portal inflammation and liver fibrosis. Moreover, the consequences of therapy on long-term sickness and death are uncertain since follow-up was not included in the aforementioned investigation. Additionally, no studies on the impact of vitamin E in NASH participants with hyperglycemia, severe fibrosis, or cirrhosis had been conducted (Al-Busafi et al., Citation2012).
Which dietary patterns influence NAFLD?
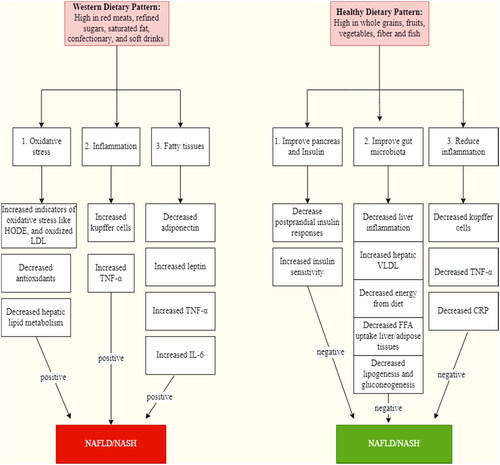
From the standpoint of dietary patterns, some supplementary research assessed the relationship between nutrition and non-alcoholic fatty liver disease (Kontogianni et al., Citation2014; Oddy et al., Citation2013). A review of these researches revealed a potential link between NAFLD and the Western food pattern. In reality, popular items in the Western dietary pattern, such as refined sugars and lipids, white bread, refined cereals, carbonated beverages, and confectionery, can quickly boost postprandial plasma glucose and insulin levels by providing extra calories and excessive quantities of sugars like fructose (Dekker et al., Citation2010). Several types of research revealed a strong link between the Western food pattern and obesity (Ritchie et al., Citation2007). When administered a similar diet to lean individuals, obese people who followed a Western eating pattern exhibited a higher incidence of de novo lipogenesis, one of the drivers of NAFLD (Schwarz et al., Citation2003). Depending on these observations and other research on the etiology, risk factors, and various dietary patterns associated with NAFLD (Abenavoli & Peta Citation2014; Arslan, Citation2014; Casas et al., Citation2014; Goel et al., Citation2014; Kong et al., Citation2014; Wenfeng et al., Citation2014), we may recommend .
Figure 4. Consequences of western and healthy dietary patterns on the onset of non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver disease (NAFLD). HODE: hydroxyoctadecadienoic acids; oxidized LDL: lipid oxidation products; TNF: tuber necrosis factor; IL-6: Interleukin 6; VLDL: very low density lipoproteins; FFA: free fatty acids; CRP: C-reactive proteins.
A review of the research (Kastorini et al., Citation2011; Puppala et al., Citation2013) also exposed that insulin resistance is negatively correlated with Mediterranean diet adherence owing to the elevated content of fruits and vegetables. Additionally, this diet promotes insulin sensitivity and hepatic steatosis in NAFLD patients and has preventive properties against metabolic syndrome and its comorbidities (Han et al., Citation2021; Ryan et al., Citation2013).
Mediterranean diet (MedDiet) and NAFLD
The most extensively researched diet plan in scientific literature, the MedDiet has been shown to provide several additional health benefits in addition to helping people lose weight, including improvements in inflammatory and oxidative stress markers, lipid and glucose metabolism, insulin sensitivity, antithrombotic and endothelial function, and insulin sensitivity (Ditano-Vázquez et al., Citation2019; Katsiki et al., Citation2021; Schwingshackl et al., Citation2020). Certain dietary components, such as MUFA (found mostly in olive oil), PUFA (present in fish, seeds, and nuts), and plant-based foods (fruits, vegetables, and legumes) have been widely credited with the positive effects of the MedDiet. Reduced risks of cancer, metabolic disorders, cardiovascular morbidity and death, and all-cause mortality have been linked to increased adherence to the MedDiet (Schwingshackl et al., Citation2020). MedDiet is a comprehensive nutritional strategy that is being explored for its potential to prevent degenerative and cardiometabolic disorders (Gotsis et al., Citation2015). It has been demonstrated that following the MedDiet in this situation can reduce the chance of getting NAFLD (Vancells Lujan et al., Citation2021).
As previously reported, a number of randomized and cross-sectional clinical testing have been carried out to evaluate the effectiveness of MedDiet in NAFLD/NASH patients (Anania et al., Citation2018; Gosal et al., Citation2021). The studies indicated that MedDiet decreased fasting glucose, lipids, BMI, blood pressure, oxidative stress, inflammation, and insulin resistance in addition to the NAFLD score, liver tests, stiffness, fibrosis, intrahepatic fat content, hepatic steatosis, and the severity and development of NAFLD (Plaz Torres et al., Citation2019; Saavedra et al., Citation2021). Notably, MedDiet’s hepatometabolic advantages occur regardless of weight reduction (Zelber-Sagi et al., Citation2017). The MedDiet has been demonstrated to help with weight, steatosis, insulin opposition, dyslipidemia, liver tests, and NAFLD severity indicators in meta-analyses including NAFLD patients. It has also been proven to reduce overall mortality and morbidity (Asbaghi et al., Citation2020; Akhlaghi et al., Citation2020; Kawaguchi et al., Citation2021; Moosavian et al., Citation2020).
According to the information above, MedDiet is advised for people with NAFLD/NASH as it can reduce hepatic steatosis and many cardiovascular risk factors, hence lowering the morbidity and death rate from CV disease (Pugliese et al., Citation2021). The National Institute for Health and Care Excellence (NICE) (Glen et al., Citation2016) the European Association for the Study of the Liver (EASL), the European Association for the Study of Diabetes (EASD), and the European Association for the Study of Obesity (EASO) jointly recommend the use of MedDiet in NAFLD/NASH patients, these guidelines are in strong favor of this approach (European Association for the Study of the Liver, Citation2016).
Dietary approach to stop hypertension (DASH) diet and NAFLD
The DASH diet is a desirable choice for those who are overweight or obese since it has been demonstrated to dramatically lower blood pressure, body weight, total cholesterol, LDL-C, and HbA1c. It also appears to protect against the occurrence of CVD and T2D (Chiavaroli et al., Citation2019; Soltani et al., Citation2016). Additionally, there is some proof that NAFLD patients benefit from the DASH diet (Parra-Vargas et al., Citation2020). According to a cross-sectional study of the Guangzhou Nutrition and Health Study, which is a cohort study based on a population with 3051 applicants, compliance to the DASH diet (characterized by a greater DASH diet rating) was self-reliantly related to a significantly lower incidence of NAFLD as well as reduced BMI, insulin resistance, and inflammatory levels (Xiao et al., Citation2020). The Multiethnic Cohort (MEC) [n = 2959 NAFLD cases (509 with cirrhosis) and 29,292 controls] and 102 NAFLD patients (identified by transient elastography) and 204 controls (Hekmatdoost et al., Citation2016) case-control study also showed a comparable negative correlation between the likelihood of NAFLD and the DASH diet (Park et al., Citation2020). In the subsequent investigation, NAFLD patients showed an even stronger inverse correlation than those without cirrhosis (Park et al., Citation2020). In addition to the risk of NAFLD, cross-sectional research including 136 people who were not smokers and did not have diabetes found an inverse relationship between the DASH score and liver fat content as determined by MRI (Watzinger et al., Citation2020).
Ketogenic diet and NAFLD
It has been shown that low-carb ketogenic diets (KD) dramatically lower intrahepatic TG levels in obese people in less than 48 h (Kirk et al., Citation2009). In addition to causing weight reduction, ketone diets (KD) may also have positive effects on liver disease by moderating inflammation and fibrosis, decreasing insulin levels, and lipogenesis. They may also boost fatty acid oxidation because of their extremely low carbohydrate content (Watanabe et al., Citation2020). Additionally, better hepatic mitochondrial redox status and lower hepatic insulin resistance have been associated with KD-related NAFLD reversal. These factors encourage the total hydrolysis of intrahepatic TG and, therefore, the production of ketones from the resulting fatty acids (Luukkonen et al., Citation2020).
In the first open-label, randomized controlled trial run, with 18 obese women with PCOS, the keto diet for 12 weeks was better than the group under control (who received Essentiale plus Yasmin, i.e. conventional drug treatment) in lowering alanine transaminase (ALT) and aspartate aminotransferase (AST) and enhancing the liver ultrasound characteristics (Li et al., Citation2021). Additionally, after two months of intervention, 39 obese individuals who followed a very low-calorie KD had higher decreases in body mass, visceral fat, and amount of fat in the liver than those who followed a normal low-calorie diet (Cunha et al., Citation2020). The authors proposed that KD-induced weight reduction and the quick mobilization of hepatic fat might be a good substitute for treating NAFLD (Cunha et al., Citation2020). Additionally, compared to pre-menopausal women, obese males may benefit more from extremely low-carb KD in terms of reducing liver test results and body weight; however, these advantages diminish after menopause (D’Abbondanza et al., Citation2020).
Intermittent fasting diet and NAFLD
A low-carb, high-fat (LCHF) diet, an intermittent calorie restraint diet (the 5:2 diet), or general lifestyle counseling (standard of care; SoC) were the treatments given to 74 patients with non-alcoholic fasting diabetes (NAFLD), as established by transient elastography, CT, MRI, or ultrasound, in a 1:1:1 open-label randomized controlled trial. The patients were then followed up for a period of 12 weeks (Holmer et al., Citation2021). Notably, accomplices in the 5:2 group were told to utilize 600 kcal/d for males and 6500 kcal/d for women on the two days each week that were not consecutive. Regarding modifications to body weight and the decrease of hepatic steatosis (as determined by MRS), both the 5:2 and LCHF diets performed better than the SoC therapy (Holmer et al., Citation2021). Additionally, the 5:2 diet was better tolerated than the LCHF diet and decreased liver hardness and LDL-C, but not the LCHF diet (Holmer et al., Citation2021).
In a prospective observational study, repeated fasting was also demonstrated to significantly lower FLI in 697 participants with or without T2D; the advantage was larger in T2D patients (Drinda et al., Citation2019). Significant reductions were detected in liver enzymes, fasting glucose, waist circumference, body weight, BMI, and HbA1c during intermittent fasting. The amount of BMI decrease and the number of fasting days were connected with FLI improvement (Drinda et al., Citation2019). Nearly 50% of the members with reference FLI ≥60, which is indicative of NAFLD, moved to a minimal FLI risk group after fasting, indicating a reversal of liver disease. The period of fasting (Midday: 250 mL of vegetable soup or fruit juice; evening: 250 mL of vegetable broth; optional: 20 g of honey) was followed by a low-calorie transition day (600 kcal a day mono diet of rice, oats, fruits, and vegetables) and, lastly, progressively reintroducing food (Food for lacto-ovo-vegetarians increasing daily by 800–1800 kcal for a minimum of three days). It ought to be mentioned that intermittent fasting was employed in this way (Drinda et al., Citation2019). Participants were instructed to consume at least ≥2 L of water each day.
In animal models of MetS, alternate-day fasting has greatly reduced overall cholesterol levels, insulin resistance, liver weight, ALT, and LDL-C (Gamil et al., Citation2021). Similarly, after 12 weeks of alternate-day fasting or time-restricted meals, 271 individuals with NAFLD (as determined by ultrasonography) revealed significantly decreased TG, total cholesterol, fat mass, and body weight (Cai et al., Citation2019). Another randomized clinical research found that an 8-week alternate-day calorie restriction program significantly lowered ALT, BMI, liver fibrosis scores, and liver steatosis grades when compared to a regular diet in 43 individuals with nonalcoholic fatty liver disease (NAFLD), which is indicated by increased liver tests (Johari et al., Citation2019). Throughout the trial, there was good adherence to the alternate-day diet, and it was well endured (Johari et al., Citation2019).
Vegetarian (vegan) diet and NAFLD
Even after accounting for age, gender, education, smoking, and alcohol use, a cross-sectional study including 1273 vegetarians and 2127 nonvegetarians (all healthy volunteers) demonstrated that the likelihood of fatty liver (as determined by ultrasound) was significantly reduced (by 21%) when comparing vegetarians to non-vegetarians (Chiu et al., Citation2018). BMI, however, somewhat mitigated this relationship. Moreover, the fibrosis score of nonvegetarians was higher than that of vegetarians (Chiu et al., Citation2018). According to the scientists, the vegetarian diet’s demonstrated advantages for the liver might be related to its high polyphenol content, which can reduce oxidative stress, inflammation, and insulin resistance and impede the advancement of nonalcoholic fatty liver disease (Chiu et al., Citation2018).
Dietary modification and management
A healthy and adequate diet is one of the essential components of nutritional quality that aids in the prevention of numerous illnesses, particularly NAFLD. The chief reason of NAFLD is poor nutrition that is low in vitamins and fiber and heavy in saturated fats and triglycerides, simple carbohydrates, xenobiotics, or environmental contaminants (Duarte et al., Citation2019). For example, by supplying more calories and vast quantities of sweets like fructose, typical items in the Western dietary pattern counting white bread, refined grains, carbonated beverages, & confectionery can fast increase postprandial plasma glucose and insulin concentrations. Obese persons with a Western diet had higher rates of de novo lipogenesis, one of the reasons of NAFLD.
The fundamental treatment for NAFLD in both children and adolescents continues to be lifestyle modification with an emphasis on wholesome eating, weight management where necessary, and frequent exercising (Neuschwander-Tetri, Citation2017). There is strong significant proof that losing weight not only lessens the amount of surplus fat in the liver but that it could also direct to the histological settlement of NASH with just a 5% weight reduction and the recurrence of fibrosis in more than half of 50% of patients with a 10% weight reduction. During a trial setting, only 10% of patients dropped 10% of their body weight, indicating that the majority of patients consider it challenging to alter their lives. Clients should receive a dietician assessment and details on nearby service organizations for weight reduction.
The majority of NASH sufferers who undergo bariatric surgery experience decreased liver steatosis, hepatocyte necrosis, and inflammation, with less obvious benefits on fibrosis. Contrarily, fibrosis tends to be improved by weight loss or lifestyle changes, and no particular diet (apart from calorie reduction) seems to be particularly helpful in NASH (Portillo-Sanchez & Cusi, Citation2016). The US Food and Drug Administration has authorized the weight-loss medications orlistat and sibutramine (FDA). Enteric lipase is inhibited by orlistat. Sibutramine, a transporter inhibitor of serotonin and norepinephrine, stimulates thermogenesis to promote satisfaction and raise calorie expenditure (Lam andYounossi, n.d.).
Important data regarding the effects of lifestyle triggered weight reduction on liver histology was obtained from a single-arm clinical experiment involving 261 sick people with biopsy-proven NASH who undertook recurring liver biopsies after 12 months of following a reduced fat hypocaloric eating plan (750 kcal less than the recommended daily intake) and 200 minutes per week of stumpy strength exercise (walking). The chance of NASH cure and fibrosis progression was correlated with the amount of weight reduction. The relationship between weight reduction and NAFLD recovery was subsequently enabled by a 12-month randomized clinical study that coupled a low-fat, low-glycemic index diet recommended by the American Dietetic Association with 90–150 minutes per week of moderate exercising (Hallsworth & Adams, Citation2019).
The liver enzyme thresholds and hepatic steatosis of patients who adhered to a restricted diet (25 calories/kg of perfect body weight) and workout program for three months were lower than those of control respondents, though it is unidentified whether hepatic inflammation and fibrosis gotten better over time. Fast weight loss brought on by a very low-energy diet (500 kcal/d) or jejunoileal bypass must be prevented since the hazard of escalating inflammation and fibrosis. To reduce 10% of your body weight in six months is a fair objective (Adams et al., Citation2005).
Furthermore, several research has shown that normalizing liver performance and reducing hepatic fat concentration may be achieved without decreasing BMI or reducing weight (Worm, Citation2020). Reducing the threshold for individual visceral adiposity that results in pancreas and liver impairment, seems to be more important. Irrespective of the degree of BMI decline, significant and medically significant decreases in liver fat has been accomplished with low-calorie diets that use meal substitutes and low-carb Mediterranean-style diets. The advantages and disadvantages of several dietary approaches are mentioned in the next paragraphs, leading to a theoretically ‘perfect’ integrative nutritional approach to reduce or perhaps yet eliminate NAFLD (Worm, Citation2020).
Based on a newly released 18-month randomized controlled intervention trial which looked at the consequences of isocaloric Mediterranean reduced carbohydrate vs. reduced fat diets in a cohort of overweight and obese individuals, the Mediterranean reduced carbohydrate diet prompted noticeably larger cutbacks in the liver, pancreas, and pericardium fat stores as well as additional prominent advancements in cardiometabolic threat indicators like triglycerides. Both diets led to an identical loss of weight, and even when weight loss was taken into account, the differences in fat deposit levels were still noticeable (Worm, Citation2020).
Additionally, a long-term investigation of a ketogenic diet in people with type 2-diabetes, who encompass a greater chance of acquiring NAFLD, was just posted (Worm, Citation2020). Participants were instructed to limit their consumption of carbohydrates until they attained nutritional ketosis (a metabolic state determined by blood levels of -hydroxybutyrate). This digitally assisted ad libitum but carbohydrate-restricted approach considerably abridged surrogate steatosis and fibrosis scores at 1–2 years. After receiving therapy for a year, there was a 20% decrease in the percentage of patients with steatosis, and there were significant increases in the percentage of patients who showed no symptoms of fibrosis (from 18% at baseline to 33%) (Worm, Citation2020).
Meal replacement plans that have been carefully devised are both nutritionally appropriate and extremely secure. The 8–12-week period of total diet substitution (no scheduled meals, snacks, or beverages) is intended to aid abstention from the temptations of typical foods and dietary behavior to promote mental transformation (Worm, Citation2020). This appears to be a key feature in adherence maintenance and considerable weight reduction. Divergent to the general principle, meal replacement programs are less likely to fail than traditional calorie-restricted diets. German guidelines for the management of obesity advise using formula diets, which are complete meal replacements providing 800–1200 kcal/d for adults with a BMI of 30 kg/m2 or above for utmost of 12 weeks (Worm, Citation2020). As a result, eating behavior modifications and weight management are essential components of NAFLD treatment.
Conclusion
Non Alcoholic fatty liver disease is becoming very general among the populations having increased rate of obesity and diabetes. Particularly diabetic patients are more prone to this disease due to defective liver metabolism; however, this malady can be regulated by following a healthy life style and dietary patterns. In the light of highlighted facts in this manuscript it is found that higher consumption of ‘healthy dietary patterns’ like fruit and vegetables, nuts, whole grains, olives, reduced fat dairy products, fiber, and fish are linked to lower risk of NAFLD, whereas high consumption of fast food, carbonated beverages, confectionary, high fat milk and milk products, trans fats, saturated fats, salty foods, refined sugars and refined grains that is ‘western dietary patterns’ is linked to a elevated threat of NAFLD. These findings are helpful for vital stakeholders in the diagnosis and treatment of NAFLD and to further avoid the complications of this disease.
Furthermore, hepatic steatosis may be improved by a high-quality diet. A useful method for controlling NAFLD is to combine physical exercise and a low-sugar diet with a Mediterranean, DASH, ketogenic, intermittent fasting, or vegan diet. In this article, specific dietary suggestions are included.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
Additional information
Funding
References
- Abenavoli, L., & Peta, V. (2014). Role of adipokines and cytokines in non-alcoholic fatty liver disease. Reviews on Recent Clinical Trials, 9(3), 1–18. https://doi.org/10.2174/1574887109666141216102458
- Adams, L. A., Angulo, P., & Lindor, K. D. (2005). Nonalcoholic fatty liver disease. CMAJ, 172(7), 899–905. https://doi.org/10.1503/cmaj.045232
- Akhlaghi, M., Ghasemi-Nasab, M., & Riasatian, M. (2020). Mediterranean diet for patients with non-alcoholic fatty liver disease, a systematic review and meta-analysis of observational and clinical investigations. Journal of Diabetes and Metabolic Disorders, 19(1), 575–584. https://doi.org/10.1007/s40200-019-00475-2
- Al-Busafi, S. A., Bhat, M., Wong, P., Ghali, P., & Deschenes, M. (2012). Antioxidant therapy in nonalcoholic steatohepatitis. Hepatitis Research and Treatment, 2012, 947575–947578. https://doi.org/10.1155/2012/947575
- Amengual, J., Ribot, J., Bonet, M. L., & Palou, A. (2010). Retinoic acid treatment enhances lipid oxidation and inhibits lipid biosynthesis capacities in the liver of mice. Cellular Physiology and Biochemistry, 25(6), 657–666. https://doi.org/10.1159/000315085
- Anania, C., Perla, F. M., Olivero, F., Pacifico, L., & Chiesa, C. (2018). Mediterranean diet, and nonalcoholic fatty liver disease. World Journal of Gastroenterology, 24(19), 2083–2094. https://doi.org/10.3748/wjg.v24.i19.2083
- Arroyave-Ospina, J. C., Wu, Z., Geng, Y., & Moshage, H. (2021). Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: Implications for prevention and therapy. Antioxidants, 10(2), 174. https://doi.org/10.3390/antiox10020174
- Arslan, N. (2014). Obesity, fatty liver disease, and intestinal microbiota. World Journal of Gastroenterology, 20(44), 16452–16463. https://doi.org/10.3748/wjg.v20.i44.16452
- Asbaghi, O., Choghakhori, R., Ashtary-Larky, D., & Abbasnezhad, A. (2020). Effects of the Mediterranean diet on cardiovascular risk factors in non-alcoholic fatty liver disease patients: a systematic review and meta-analysis. Clinical Nutrition ESPEN, 37, 148–156. https://doi.org/10.1016/j.clnesp.2020.03.003
- Banini, B. A., & Sanyal, A. J. (2019). Vitamin E in Nonalcoholic Fatty Liver Disease. In: Weber, P., Birringer, M., Blumberg, J., Eggersdorfer, M., Frank, J. (Eds.), Vitamin E in human health. nutrition and health. Humana Press. https://doi.org/10.1007/978-3-030-05315-4_23
- Berná, G., & Romero‐Gomez, M. (2020). The role of nutrition in non‐alcoholic fatty liver disease: pathophysiology and management. Liver International, 40(S1), 102–108. https://doi.org/10.1111/liv.14360
- Brøns, C., Jensen, C. B., Storgaard, H., Hiscock, N. J., White, A., Appel, J. S., Jacobsen, S., Nilsson, E., Larsen, C. M., Astrup, A., Quistorff, B., & Vaag, A. (2009). Impact of short‐term high‐fat feeding on glucose and insulin metabolism in young healthy men. The Journal of Physiology, 587(Pt 10), 2387–2397. https://doi.org/10.1113/jphysiol.2009.169078
- Browning, J. D., Baker, J. A., Rogers, T., Davis, J., Satapati, S., & Burgess, S. C. (2011). Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. The American Journal of Clinical Nutrition, 93(5), 1048–1052. https://doi.org/10.3945/ajcn.110.007674
- Cai, H., Qin, Y.-L., Shi, Z.-Y., Chen, J.-H., Zeng, M.-J., Zhou, W., Chen, R.-Q., & Chen, Z.-Y. (2019). Effects of alternate-day fasting on body weight and dyslipidemia in patients with non-alcoholic fatty liver disease: a randomized controlled trial. BMC Gastroenterology, 19(1), 219. https://doi.org/10.1186/s12876-019-1132-8
- Cañete, N. G., & Agüero, S. D. (2014). Soya isoflavones and evidences on cardiovascular protection. Nutricion Hospitalaria, 29(6), 1271–1282.
- Casas, R., Sacanella, E., & Estruch, R. (2014). The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocrine, Metabolic & Immune Disorders Drug Targets, 14(4), 245–254. https://doi.org/10.2174/1871530314666140922153350
- Cherkaoui-Malki, M., Surapureddi, S., El-Hajj, H. I., Vamecq, J., & Andreoletti, P. (2012). Hepatic steatosis and peroxisomal fatty acid beta-oxidation. Current Drug Metabolism, 13(10), 1412–1421. https://doi.org/10.2174/138920012803762765
- Chiavaroli, L., Viguiliouk, E., Nishi, S. K., Blanco Mejia, S., Rahelić, D., Kahleová, H., Salas-Salvadó, J., Kendall, C. W., & Sievenpiper, J. L. (2019). DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients, 11(2), 338. https://doi.org/10.3390/nu11020338
- Chiu, T. H., Lin, M. N., Pan, W. H., Chen, Y. C., & Lin, C. L. (2018). Vegetarian diet, food substitution, and nonalcoholic fatty liver. Tzu-Chi Medical Journal, 30(2), 102–109. https://doi.org/10.4103/tcmj.tcmj_109_17
- Cortez-Pinto, H., Jesus, L., Barros, H., Lopes, C., Moura, M. C., & Camilo, M. E. (2006). How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clinical Nutrition, 25(5), 816–823. https://doi.org/10.1016/j.clnu.2006.01.027
- Cunha, G. M., Guzman, G., Correa De Mello, L. L., Trein, B., Spina, L., Bussade, I., Marques Prata, J., Sajoux, I., & Countinho, W. (2020). Efficacy of a 2-month Very Low-Calorie Ketogenic Diet (VLCKD) compared to a standard low-calorie diet in reducing visceral and liver fat accumulation in patients with obesity. Frontiers in Endocrinology, 11, 607. https://doi.org/10.3389/fendo.2020.00607
- D’Abbondanza, M., Ministrini, S., Pucci, G., Nulli Migliola, E., Martorelli, E.-E., Gandolfo, V., Siepi, D., Lupattelli, G., & Vaudo, G. (2020). Very low-carbohydrate ketogenic diet for the treatment of severe obesity and associated nonalcoholic fatty liver disease: the role of sex differences. Nutrients, 12(9), 2748. https://doi.org/10.3390/nu12092748
- Dekker, M. J., Su, Q., Baker, C., Rutledge, A. C., & Adeli, K. (2010). Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. American Journal of Physiology, Endocrinology and Metabolism, 299(5), E685–94. https://doi.org/10.1152/ajpendo.00283.2010
- Dhibi, M., Brahmi, F., Mnari, A., Houas, Z., Chargui, I., Bchir, L., Gazzah, N., Alsaif, M. A., & Hammami, M. (2011). The intake of high fat diet with different trans fatty acid levels differential induces oxidative stress and non-alcoholic fatty liver disease (NAFLD) in rats. Nutrition & Metabolism, 8(1), 65. https://doi.org/10.1186/1743-7075-8-65
- Ditano-Vázquez, P., Torres-Peña, J. D., Galeano-Valle, F., Pérez-Caballero, A. I., Demelo-Rodríguez, P., Lopez-Miranda, J., Katsiki, N., Delgado-Lista, J., & Alvarez-Sala-Walther, L. A. (2019). The fluid aspect of the Mediterranean diet in the prevention and management of cardiovascular disease and diabetes: the role of polyphenol content in moderate consumption of wine and olive oil. Nutrients, 11(11), 2833. https://doi.org/10.3390/nu11112833
- Drinda, S., Grundler, F., Neumann, T., Lehmann, T., Steckhan, N., Michalsen, A., & Wilhelmi de Toledo, F. (2019). Effects of periodic fasting on fatty liver index – A prospective observational study. Nutrients, 11(11), 2601. https://doi.org/10.3390/nu11112601
- Duarte, S. M. B., Stefano, J. T., Vanni, D. S., Carrilho, F. J., & Oliveira, C. P. M. S. (2019). Impact of current diet at the risk of non-alcoholic fatty liver disease (NAFLD). Arquivos de Gastroenterologia, 56(4), 431–439. https://doi.org/10.1590/S0004-2803.201900000-67
- Dyson, J. K., Anstee, Q. M., & McPherson, S. (2015). Republished: Non-alcoholic fatty liver disease: a practical approach to treatment. Postgraduate Medical Journal, 91(1072), 92–101. https://doi.org/10.1136/postgradmedj-2013-100404rep
- European Association for the Study of the Liver. (2016). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of Hepatology, 64:1388–1402.
- Fan, J.-G., & Cao, H.-X. (2013). Role of diet and nutritional management in non-alcoholic fatty liver disease: Dietary management in fatty liver. Journal of Gastroenterology and Hepatology, 28(S4), 81–87. https://doi.org/10.1111/jgh.12244
- Foster, T., Budoff, M. J., Saab, S., Ahmadi, N., Gordon, C., & Guerci, A. D. (2011). Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. The American Journal of Gastroenterology, 106(1), 71–77. https://doi.org/10.1038/ajg.2010.299
- Galisteo, M., Duarte, J., & Zarzuelo, A. (2008). Effects of dietary fibers on disturbances clustered in the metabolic syndrome. The Journal of Nutritional Biochemistry, 19(2), 71–84. https://doi.org/10.1016/j.jnutbio.2007.02.009
- Gamil, N. M. B., El Agaty, S. M., Megahed, G. K., Mansour, R. S., & Abdel-Latif, M. S. (2021). Reversion to a regular diet with alternate day fasting can cure grade-I non-alcoholic fatty liver disease (NAFLD) in high fructose-intake-associated metabolic syndrome. Egyptian Liver Journal, 11, 60.
- Glen, J., Floros, L., Day, C., & Pryke, R. (2016). Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ, 354, i4428. https://doi.org/10.1136/bmj.i4428
- Goel, A., Gupta, M., & Aggarwal, R. (2014). Gut microbiota and liver disease. Journal of Gastroenterology and Hepatology, 29(6), 1139–1148. https://doi.org/10.1111/jgh.12556
- Gorman, S., Black, L., Feelisch, M., Hart, P., & Weller, R. (2015). Can Skin Exposure to Sunlight Prevent Liver Inflammation? Nutrients, 7(5), 3219–3239. https://doi.org/10.3390/nu7053219
- Gosal, H., Kaur, H., Chakwop Ngassa, H., Elmenawi, K. A., Anil, V., & Mohammed, L. (2021). The significance of the Mediterranean diet in the management of non-alcoholic fatty liver disease: a systematic review. Cureus, 13(6), e15618. https://doi.org/10.7759/cureus.15618
- Gotsis, E., Anagnostis, P., Mariolis, A., Vlachou, A., Katsiki, N., & Karagiannis, A. (2015). Health benefits of the Mediterranean Diet: an update of research over the last 5 years. Angiology, 66(4), 304–318. https://doi.org/10.1177/0003319714532169
- Hallberg, S. J., McKenzie, A. L., Williams, P. T., Bhanpuri, N. H., Peters, A. L., Campbell, W. W., Hazbun, T. L., Volk, B. M., McCarter, J. P., Phinney, S. D., & Volek, J. S. (2018). Effectiveness and safety of a novel care model for the management of type 2 diabetes at 1 year: an open-label, non-randomized, controlled study. Diabetes Therapy, 9(2), 583–612. https://doi.org/10.1007/s13300-018-0373-9
- Hallsworth, K., & Adams, L. A. (2019). Lifestyle modification in NAFLD/NASH: Facts and figures. JHEP Reports, 1(6), 468–479. https://doi.org/10.1016/j.jhepr.2019.10.008
- Han, M. A. T., Yu, Q., Tafesh, Z., & Pyrsopoulos, N. (2021). Diversity in nafld: A review of manifestations of nonalcoholic fatty liver disease in different ethnicities globally. Journal of Clinical and Translational Hepatology, 9(1), 71–80. https://doi.org/10.14218/JCTH.2020.00082
- Hardy, T., Anstee, Q. M., & Day, C. P. (2015). Nonalcoholic fatty liver disease: New treatments. Current Opinion in Gastroenterology, 31(3), 175–183. https://doi.org/10.1097/MOG.0000000000000175
- Hassan, M., Shah, Y., Tariq, A., Qasim, Z., Jabbar, S., & Fatima, M. (2021). A widespread acquired ailment, nonalcoholic fatty liver disease; its approaches related to nutritional therapy. Acta Scientifci Nutritional Health, 5(10), 36–50. https://doi.org/10.31080/ASNH.2020.05.0930
- Hassani Zadeh, S., Mansoori, A., & Hosseinzadeh, M. (2020). Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. Journal of Gastroenterology and Hepatology, 36(6), 1470–1478. https://doi.org/10.1111/jgh.15363
- Haufe, S., Engeli, S., Kast, P., Böhnke, J., Utz, W., Haas, V., Hermsdorf, M., Mähler, A., Wiesner, S., Birkenfeld, A. L., Sell, H., Otto, C., Mehling, H., Luft, F. C., Eckel, J., Schulz-Menger, J., Boschmann, M., & Jordan, J. (2011). Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology, 53(5), 1504–1514. https://doi.org/10.1002/hep.24242
- Havel, P. J. (2005). Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutrition Reviews, 63(5), 133–157. https://doi.org/10.1111/j.1753-4887.2005.tb00132.x
- Hekmatdoost, A., Shamsipour, A., Meibodi, M., Gheibizadeh, N., Eslamparast, T., & Poustchi, H. (2016). Adherence to the dietary approaches to stop hypertension (DASH) and risk of nonalcoholic fatty liver disease. International Journal of Food Sciences and Nutrition, 67(8), 1024–1029. https://doi.org/10.1080/09637486.2016.1210101
- Hollingsworth, K. G., Abubacker, M. Z., Joubert, I., Allison, M. E. D., & Lomas, D. J. (2006). Low-carbohydrate diet induced reduction of hepatic lipid content observed with a rapid non-invasive MRI technique. The British Journal of Radiology, 79(945), 712–715. https://doi.org/10.1259/bjr/23166141
- Holmer, M., Lindqvist, C., Petersson, S., Moshtaghi-Svensson, J., Tillander, V., Brismar, T. B., Hagström, H., & Stål, P. (2021). Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet – A randomized controlled trial. JHEP Reports, 3(3), 100256. https://doi.org/10.1016/j.jhepr.2021.100256
- Hormoznejad, R., Mohammad Shahi, M., Rahim, F., Helli, B., Alavinejad, P., & Sharhani, A. (2020). Combined cranberry supplementation and weight loss diet in non-alcoholic fatty liver disease: a double-blind placebo-controlled randomized clinical trial. International Journal of Food Sciences and Nutrition, 71(8), 991–1000. https://doi.org/10.1080/09637486.2020.1746957
- Hu, F. (2002). Dietary pattern analysis: a new direction in nutritional epidemiology. Current Opinion in Lipidology, 13(1), 3–9. https://doi.org/10.1097/00041433-200202000-00002
- Hyde, P. N., Sapper, T. N., Crabtree, C. D., LaFountain, R. A., Bowling, M. L., Buga, A., Fell, B., McSwiney, F. T., Dickerson, R. M., Miller, V. J., Scandling, D., Simonetti, O. P., Phinney, S. D., Kraemer, W. J., King, S. A., Krauss, R. M., & Volek, J. S. (2019). Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight, 4(12), e128308. https://doi.org/10.1172/jci.insight.128308
- Johari, M. I., Yusoff, K., Haron, J., Nadarajan, C., Ibrahim, K. N., Wong, M. S., Hafidz, M. I. A., Chua, B. E., Hamid, N., Arifin, W. N., Ma, Z. F., & Lee, Y. Y. (2019). A randomized controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Scientific Reports, 9(1), 11232. https://doi.org/10.1038/s41598-019-47763-8
- Kastorini, C.-M., Milionis, H. J., Esposito, K., Giugliano, D., Goudevenos, J. A., & Panagiotakos, D. B. (2011). The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534, 906 individuals. Journal of the American College of Cardiology, 57(11), 1299–1313. https://doi.org/10.1016/j.jacc.2010.09.073
- Katsiki, N., Pérez-Martínez, P., & Lopez-Miranda, J. (2021). Olive oil intake and cardiovascular disease prevention: ‘seek and you shall find’. Current Cardiology Reports, 23(6), 64. https://doi.org/10.1007/s11886-021-01496-1
- Kawaguchi, T., Charlton, M., Kawaguchi, A., Yamamura, S., Nakano, D., Tsutsumi, T., Zafer, M., & Torimura, T. (2021). Effects of Mediterranean diet in patients with nonalcoholic fatty liver disease: a systematic review meta-analysis, and meta-regression analysis of randomized controlled trials. Seminars in Liver Disease, 41(3), 225–234. https://doi.org/10.1055/s-0041-1723751
- Kawanaka, M., Mahmood, S., Niiyama, G., Izumi, A., Kamei, A., Ikeda, H., Suehiro, M., Togawa, K., Sasagawa, T., Okita, M., Nakamura, H., Yodoi, J., & Yamada, G. (2004). Control of oxidative stress and reduction in biochemical markers by Vitamin E treatment in patients with nonalcoholic steatohepatitis: a pilot study. Hepatology Research, 29(1), 39–41. https://doi.org/10.1016/j.hepres.2004.02.002
- Kawasaki, T., Igarashi, K., Koeda, T., Sugimoto, K., Nakagawa, K., Hayashi, S., Yamaji, R., Inui, H., Fukusato, T., & Yamanouchi, T. (2009). Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. The Journal of Nutrition, 139(11), 2067–2071. https://doi.org/10.3945/jn.109.105858
- Kirk, E., Reeds, D. N., Finck, B. N., Mayurranjan, M. S., Patterson, B. W., & Klein, S. (2009). Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology, 136(5), 1552–1560.
- Kirk, E., Reeds, D. N., Finck, B. N., Mayurranjan, S. M., Patterson, B. W., & Klein, S. (2009). Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology, 136(5), 1552–1560. https://doi.org/10.1053/j.gastro.2009.01.048
- Kong, L. C., Holmes, B. A., Cotillard, A., Habi-Rachedi, F., Brazeilles, R., Gougis, S., Gausserès, N., Cani, P. D., Fellahi, S., Bastard, J.-P., Kennedy, S. P., Doré, J., Ehrlich, S. D., Zucker, J.-D., Rizkalla, S. W., & Clément, K. (2014). Dietary patterns differently associate with inflammation and gut microbiota in overweight and obese subjects. PLOS One, 9(10), e109434. https://doi.org/10.1371/journal.pone.0109434
- Kontogianni, M. D., Tileli, N., Margariti, A., Georgoulis, M., Deutsch, M., Tiniakos, D., Fragopoulou, E., Zafiropoulou, R., Manios, Y., & Papatheodoridis, G. (2014). Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clinical Nutrition, 33(4), 678–683. https://doi.org/10.1016/j.clnu.2013.08.014
- Lai, H. S., Lin, W. H., Chen, P. R., Wu, H. C., Lee, P. H., & Chen, W. J. (2005). Effects of a High Fiber Diet on Hepatocyte Apoptosis and Liver Regeneration After Partial Hepatectomy in Rats With Fatty Liver. Journal of Parenteral and Enteral Nutrition, 29(6), 401–407.
- Larson-Meyer, D. E., Newcomer, B. R., Heilbronn, L. K., Volaufova, J., Smith, S. R., Alfonso, A. J., Lefevre, M., Rood, J. C., Williamson, D. A., Ravussin, E., & Team, T. P. C. (2008). Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity, 16(6), 1355–1362. https://doi.org/10.1038/oby.2008.201
- Lavine, J. E. (2000). Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. The Journal of Pediatrics, 136(6), 734–738. https://doi.org/10.1016/S0022-3476(00)05040-X
- Lavine, J. E., Schwimmer, J. B., Van Natta, M. L., Molleston, J. P., Murray, K. F., Rosenthal, P., Abrams, S. H., Scheimann, A. O., Sanyal, A. J., Chalasani, N., Tonascia, J., Ünalp, A., Clark, J. M., Brunt, E. M., Kleiner, D. E., Hoofnagle, J. H., … & Robuck, P. R. (2011). Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA, 305(16), 1659–1668. https://doi.org/10.1001/jama.2011.520
- Lê, K.-A., Ith, M., Kreis, R., Faeh, D., Bortolotti, M., Tran, C., Boesch, C., & Tappy, L. (2009). Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. The American Journal of Clinical Nutrition, 89(6), 1760–1765. https://doi.org/10.3945/ajcn.2008.27336
- Leaf, A., & Lansdowne, Z. (2014). Vitamins-conventional uses and new insights. World Review of Nutrition and Dietetics, 110, 152–166. https://doi.org/10.1159/000358464
- Lee, S.-W., Baek, S.-M., Kang, K.-K., Lee, A.-R., Kim, T.-U., Choi, S.-K., Roh, Y.-S., Hong, I.-H., Park, S.-J., Kim, T.-H., Jeong, K.-S., & Park, J.-K. (2021). Vitamin C deficiency inhibits nonalcoholic fatty liver disease progression through impaired de novo lipogenesis. The American Journal of Pathology, 191(9), 1550–1563. https://doi.org/10.1016/j.ajpath.2021.05.020
- León-Mimila, P., Villamil-Ramírez, H., Li, X. S., Shih, D. M., Hui, S. T., Ocampo-Medina, E., López-Contreras, B., Morán-Ramos, S., Olivares-Arevalo, M., Grandini-Rosales, P., Macías-Kauffer, L., González-González, I., Hernández-Pando, R., Gómez-Pérez, F., Campos-Pérez, F., Aguilar-Salinas, C., Larrieta-Carrasco, E., Villarreal-Molina, T., Wang, Z., … Canizales-Quinteros, S. (2021). Trimethylamine N-oxide levels are associated with NASH in obese subjects with type 2 diabetes. Diabetes & Metabolism, 47(2), 101183. https://doi.org/10.1016/j.diabet.2020.07.010
- Li, J., Bai, W.-P., Jiang, B., Bai, L.-R., Gu, B., Yan, S.-X., Li, F.-Y., & Huang, B. (2021). Ketogenic diet in women with polycystic ovary syndrome and liver dysfunction who are obese: a randomized, open-label, parallel-group, controlled pilot trial. The Journal of Obstetrics and Gynaecology Research, 47(3), 1145–1152. https://doi.org/10.1111/jog.14650
- Luukkonen, P. K., Dufour, S., Lyu, K., Zhang, X.-M., Hakkarainen, A., Lehtimäki, T. E., Cline, G. W., Petersen, K. F., Shulman, G. I., & Yki-Järvinen, H. (2020). Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America, 117(13), 7347–7354. https://doi.org/10.1073/pnas.1922344117
- Marchildon, F., St-Louis, C., Akter, R., Roodman, V., & Wiper-Bergeron, N. L. (2010). Transcription factor Smad3 is required for the inhibition of adipogenesis by retinoic acid. The Journal of Biological Chemistry, 285(17), 13274–13284. https://doi.org/10.1074/jbc.M109.054536
- Mardinoglu, A., Wu, H., Bjornson, E., Zhang, C., Hakkarainen, A., Räsänen, S. M., Lee, S., Mancina, R. M., Bergentall, M., Pietiläinen, K. H., Söderlund, S., Matikainen, N., Ståhlman, M., Bergh, P.-O., Adiels, M., Piening, B. D., Granér, M., Lundbom, N., Williams, K. J., … Borén, J. (2018). An integrated understanding of the rapid metabolic benefits of a carbohydrate-restricted diet on hepatic steatosis in humans. Cell Metabolism, 27(3), 559–571.e5. https://doi.org/10.1016/j.cmet.2018.01.005
- Mehta, K., Van Thiel, D. H., Shah, N., & Mobarhan, S. (2002). Nonalcoholic fatty liver disease: Pathogenesis and the role of antioxidants. Nutrition Reviews, 60(9), 289–293. https://doi.org/10.1301/002966402320387224
- Mondul, A., Mancina, R. M., Merlo, A., Dongiovanni, P., Rametta, R., Montalcini, T., Valenti, L., Albanes, D., & Romeo, S. (2015). PNPLA3 I148M variant influences circulating retinol in adults with nonalcoholic fatty liver disease or obesity. The Journal of Nutrition, 145(8), 1687–1691. https://doi.org/10.3945/jn.115.210633
- Moosavian, S. P., Arab, A., & Paknahad, Z. (2020). The effect of a Mediterranean diet on metabolic parameters in patients with non-alcoholic fatty liver disease: a systematic review of randomized controlled trials. Clinical Nutrition ESPEN, 35, 40–46. https://doi.org/10.1016/j.clnesp.2019.10.008
- Mouzaki, M., & Allard, J. P. (2012). The role of nutrients in the development, progression, and treatment of nonalcoholic fatty liver disease. Journal of Clinical Gastroenterology, 46(6), 457–467. https://doi.org/10.1097/MCG.0b013e31824cf51e
- Neuschwander-Tetri, B. A. (2017). Non-alcoholic fatty liver disease. BMC Medicine, 15(1), 45. https://doi.org/10.1186/s12916-017-0806-8
- Oddy, W. H., Herbison, C. E., Jacoby, P., Ambrosini, G. L., O’Sullivan, T. A., Ayonrinde, O. T., Olynyk, J. K., Black, L. J., Beilin, L. J., Mori, T. A., Hands, B. P., & Adams, L. A. (2013). The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. The American Journal of Gastroenterology, 108(5), 778–785. https://doi.org/10.1038/ajg.2013.95
- Ouyang, X., Cirillo, P., Sautin, Y., McCall, S., Bruchette, J. L., Diehl, A. M., Johnson, R. J., & Abdelmalek, M. F. (2008). Fructose consumption as a risk factor for non-alcoholic fatty liver disease. Journal of Hepatology, 48(6), 993–999. https://doi.org/10.1016/j.jhep.2008.02.011
- Park, S. Y., Noureddin, M., Boushey, C., Wilkens, L. R., & Setiawan, V. W. (2020). Diet quality association with nonalcoholic fatty liver disease by cirrhosis status: the multiethnic cohort. Current Developments in Nutrition, 4(3), nzaa024. https://doi.org/10.1093/cdn/nzaa024
- Parra-Vargas, M., Rodriguez-Echevarria, R., & Jimenez-Chillaron, J. C. (2020). Nutritional approaches for managing nonalcoholic fatty liver disease: an evidence-based review. Nutrients, 12(12), 3860. https://doi.org/10.3390/nu12123860
- Pirazzi, C., Valenti, L., Motta, B. M., Pingitore, P., Hedfalk, K., Mancina, R. M., Burza, M. A., Indiveri, C., Ferro, Y., Montalcini, T., Maglio, C., Dongiovanni, P., Fargion, S., Rametta, R., Pujia, A., Andersson, L., Ghosal, S., Levin, M., Wiklund, O., … Romeo, S. (2014). PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Human Molecular Genetics, 23(15), 4077–4085. https://doi.org/10.1093/hmg/ddu121
- Plaz Torres, M. C., Aghemo, A., Lleo, A., Bodini, G., Furnari, M., Marabotto, E., Miele, L., & Giannini, E. G. (2019). Mediterranean diet and NAFLD: what we know and questions that still need to be answered. Nutrients, 11(12), 2971. https://doi.org/10.3390/nu11122971
- Portillo-Sanchez, P., & Cusi, K. (2016). Treatment of non-alcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes mellitus. Clinical Diabetes and Endocrinology, 2(1), 9. https://doi.org/10.1186/s40842-016-0027-7
- Pugliese, N., Plaz Torres, M. C., Petta, S., Valenti, L., Giannini, E. G., & Aghemo, A. (2021). Is there an ‘ideal’ diet for patients with NAFLD? European Journal of Clinical Investigation, 52(3), e13659. https://doi.org/10.1111/eci.13659
- Puppala, J., Siddapuram, S. P., Akka, J., & Munshi, A. (2013). Genetics of nonalcoholic Fatty liver disease: an overview. Journal of Genetics and Genomics, 40(1), 15–22. https://doi.org/10.1016/j.jgg.2012.12.001
- Recena Aydos, L., Aparecida do Amaral, L., Serafim de Souza, R., Jacobowski, A. C., Freitas Dos Santos, E., & Rodrigues Macedo, M. L. (2019). Nonalcoholic fatty liver disease induced by high-fat diet in C57bl/6 models. Nutrients, 11(12), 3067. https://doi.org/10.3390/nu11123067
- Rehackova, L., Araújo‐Soares, V., Adamson, A. J., Steven, S., Taylor, R., & Sniehotta, F. F. (2017). Acceptability of a very‐low‐energy diet in Type 2 diabetes: patient experiences and behaviour regulation. Diabetic Medicine, 34(11), 1554–1567. https://doi.org/10.1111/dme.13426
- Ritchie, L. D., Spector, P., Stevens, M. J., Schmidt, M. M., Schreiber, G. B., Striegel-Moore, R. H., Wang, M.-C., & Crawford, P. B. (2007). Dietary patterns in adolescence are related to adiposity in young adulthood in black and white females. The Journal of Nutrition, 137(2), 399–406. https://doi.org/10.1093/jn/137.2.399
- Romero-Gómez, M., Zelber-Sagi, S., & Trenell, M. (2017). Treatment of NAFLD with diet, physical activity and exercise. Journal of Hepatology, 67(4), 829–846. https://doi.org/10.1016/j.jhep2017.05.016.
- Ryan, M. C., Itsiopoulos, C., Thodis, T., Ward, G., Trost, N., Hofferberth, S., O’Dea, K., Desmond, P. V., Johnson, N. A., & Wilson, A. M. (2013). The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. Journal of Hepatology, 59(1), 138–143. https://doi.org/10.1016/j.jhep.2013.02.012
- Saavedra, Y., Mena, V., & Priken, K. (2021). Effect of the Mediterranean diet on histological indicators and imaging tests in nonalcoholic fatty liver disease. Gastroenterologia y Hepatologia, 45(5), 350–360. https://doi.org/10.1016/j.gastrohep.2021.06.006
- Sanyal, A. J., Chalasani, N., Kowdley, K. V., McCullough, A., Diehl, A. M., Bass, N. M., Neuschwander-Tetri, B. A., Lavine, J. E., Tonascia, J., Unalp, A., Van Natta, M., Clark, J., Brunt, E. M., Kleiner, D. E., Hoofnagle, J. H., & Robuck, P. R. (2010). Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England Journal of Medicine, 362(18), 1675–1685. https://doi.org/10.1056/NEJMoa0907929
- Schwarz, J. M., Linfoot, P., Dare, D., & Aghajanian, K. (2003). Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. The American Journal of Clinical Nutrition, 77(1), 43–50. https://doi.org/10.1093/ajcn/77.1.43
- Schwingshackl, L., Morze, J., & Hoffmann, G. (2020). Mediterranean diet and health status: active ingredients and pharmacological mechanisms. British Journal of Pharmacology, 177(6), 1241–1257. https://doi.org/10.1111/bph.14778
- Senoo, H., Yoshikawa, K., Morii, M., Miura, M., Imai, K., & Mezaki, Y. (2010). Hepatic stellate cell (vitamin A‐storing cell) and its relative–past, present and future. Cell Biology International, 34(12), 1247–1272. https://doi.org/10.1042/CBI20100321
- Soltani, S., Shirani, F., Chitsazi, M. J., & Salehi-Abargouei, A. (2016). The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Obesity Reviews, 17(5), 442–454. https://doi.org/10.1111/obr.12391
- Sullivan, S. (2010). Implications of diet on nonalcoholic fatty liver disease. Current Opinion in Gastroenterology, 26(2), 160–164. https://doi.org/10.1097/MOG.0b013e3283358a58
- Uesugi, T., Froh, M., Arteel, G. E., Bradford, B. U., Wheeler, M. D., Gäbele, E., Isayama, F., & Thurman, R. G. (2002). Role of Lipopolysaccharide-Binding Protein in Early Alcohol-Induced Liver Injury in Mice. Journal of Immunology, 168(6), 2963–2969. https://doi.org/10.4049/jimmunol.168.6.2963
- Vajro, P., Mandato, C., Franzese, A., Ciccimarra, E., Lucariello, S., Savoia, M., Capuano, G., & Migliaro, F. (2004). Vitamin E treatment in pediatric obesity-related liver disease: a randomized study. Journal of Pediatric Gastroenterology and Nutrition, 38(1), 48–55. https://doi.org/10.1097/00005176-200401000-00012
- Vancells Lujan, P., Viñas Esmel, E., & Sacanella Meseguer, E. (2021). Overview of Non-Alcoholic Fatty Liver Disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients, 13(5), 1442. https://doi.org/10.3390/nu13051442
- Viljanen, A. P. M., Iozzo, P., Borra, R., Kankaanpää, M., Karmi, A., Lautamäki, R., Järvisalo, M., Parkkola, R., Rönnemaa, T., Guiducci, L., Lehtimäki, T., Raitakari, O. T., Mari, A., & Nuutila, P. (2009). Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. The Journal of Clinical Endocrinology and Metabolism, 94(1), 50–55. https://doi.org/10.1210/jc.2008-1689
- Watanabe, M., Tozzi, R., Risi, R., Tuccinardi, D., Mariani, S., & Basciani, S. (2020). Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obesity Reviews, 21, e13024.
- Watzinger, C., Nonnenmacher, T., Grafetstätter, M., Sowah, S. A., Ulrich, C. M., Kauczor, H.-U., Kaaks, R., Schübel, R., Nattenmüller, J., & Kühn, T. (2020). Dietary factors in relation to liver fat content: a cross-sectional study. Nutrients, 12(3), 825. https://doi.org/10.3390/nu12030825
- Wenfeng, Z., Yakun, W., Di, M., Jianping, G., Chuanxin, W., & Chun, H. (2014). Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Annals of Hepatology. 13(5), 489–495. https://doi.org/10.1016/S1665-2681(19)31247-5
- Westerbacka, J., Lammi, K., Häkkinen, A.-M., Rissanen, A., Salminen, I., Aro, A., & Yki-Järvinen, H. (2005). Dietary fat content modifies liver fat in overweight nondiabetic subjects. The Journal of Clinical Endocrinology and Metabolism, 90(5), 2804–2809. https://doi.org/10.1210/jc.2004-1983
- Worm, N. (2020). Beyond body weight-loss: Dietary strategies targeting intrahepatic fat in NAFLD. Nutrients, 12(5), 1316. https://doi.org/10.3390/nu12051316
- Xiao, M.-L., Lin, J.-S., Li, Y.-H., Liu, M., Deng, Y.-Y., Wang, C.-Y., & Chen, Y.-M. (2020). Adherence to the dietary approaches to stop hypertension (DASH) diet is associated with a lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public Health Nutrition, 23(4), 674–682. https://doi.org/10.1017/S1368980019002568
- Yasutake, K., Kohjima, M., Kotoh, K., Nakashima, M., Nakamuta, M., & Enjoji, M. (2014). Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World Journal of Gastroenterology, 20(7), 1756–1767. https://doi.org/10.3748/wjg.v20.i7.1756
- Yu, L., Yuan, M., & Wang, L. (2017). The effect of omega-3 unsaturated fatty acids on non-alcoholic fatty liver disease: A systematic review and meta-analysis of RCTs. Pakistan Journal of Medical Sciences, 33(4), 1022–1028. https://doi.org/10.12669/pjms.334.12315
- Zelber-Sagi, S., Salomone, F., & Mlynarsky, L. (2017). The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver International, 37(7), 936–949. https://doi.org/10.1111/liv.13435
- Zöhrer, E., Alisi, A., Jahnel, J., Mosca, A., Della Corte, C., Crudele, A., Fauler, G., & Nobili, V. (2017). Efficacy of docosahexaenoic acid–choline–vitamin E in paediatric NASH: a randomized controlled clinical trial. Applied Physiology, Nutrition, and Metabolism, 42(9), 948–954. https://doi.org/10.1139/apnm-2016-0689

