?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Erythrina stricta Roxb., an underutilized legume species native to the Indian subcontinent, is traditionally employed in various medicinal applications. This study systematically examines the nutritional quality, encompassing proximate and mineral composition of E. stricta seeds, with a focus on characterizing the seed oil. The seeds exhibit commendable proximate composition, with 26.81% protein and 18.71% fibre. Noteworthy mineral elements include 5.0 mg/g DW of calcium and 787.0, 32.7, 36.8 and 497.0 µg/g of iron, copper, boron and zinc, respectively. The seeds yield 13.43% oil, with oleic, palmitic, linoleic and stearic acids as prominent fatty acids, constituting 48.82%, 20.63%, 20.27% and 6.47%, respectively. Antinutrients such as oxalate and phytate are present in concentrations of 26.85 and 16.04 mg/g FW, respectively. In conclusion, this study underscores E. stricta seeds as a robust source of both nutrients and oil, warranting further exploration and consideration for potential applications.
Reviewing editor:
Subjects:
1. Introduction
The genus Erythrina L. belongs to the family Fabaceae and encompasses 123 species, predominantly found in tropical regions (POWO, Citation2023a). The species of Erythrina are renowned for their medicinal properties and are utilized in traditional medicine to treat parasitic and microbial infections, inflammation, cancer, wounds and other ailments (Paterson, Citation1994). The genus reportedly holds an array of phytochemicals, including xanthones, tannins, triterpenoids, saponins, phenols, flavonoids, steroids and catechins, accountable for the activities mentioned above (Rambo et al., Citation2019). Additionally, Erythrina is well-known for its bioactive tetracyclic alkaloids, known as erythrinan alkaloids (Rambo et al., Citation2019; Fahmy et al., Citation2020).
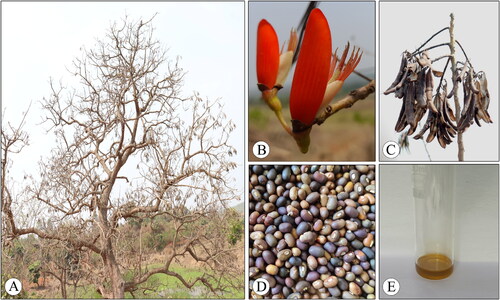
Legumes are a rich source of nutrients, particularly proteins, oil, potassium and fiber, with a low glycaemic index. Most legumes are either wild or semi-domesticated, allowing seasonal harvesting by local communities. Their resilience to drought and efficient nitrogen fixation contributes to elevated crop yield and food production (Samtiya et al., Citation2020; Ayilara et al., Citation2022). This capacity of legumes presents an opportunity to explore new legume crops to address poverty and malnutrition, especially in our world with a rapidly growing population (Murthy & Paek, Citation2021). Therefore, exploring the full potential of Erythrina species that are being consumed as a nutritional source in various regions of the world is imperative. Species such as E. variegata, E. abyssinica, E. arborescens and E. corallodendron are extensively used as fodder for cattle (Paterson, Citation1994), while the seeds of E. variegata and E. edulis serve as a nutritional source for humans (Lim, Citation2014; Vilcanqui-Pérez et al., Citation2022). However, many species within the Erythrina genus remain unexplored concerning their nutraceutical value, chemical composition and medicinal attributes, with Erythrina stricta Roxb. being a notable example. E. stricta is popularly known as the ‘Prickly Coral Tree’ or ‘Indian Coral Tree’ which is deciduous, 7–12 tall (), branches have short whitish prickles. Flowers are borne on raceme about 15 cm, with flowers arranged in clusters of 3–4 (). Flowers are red in colour and attractive, since this plant bears prickles it is called the prickly coral tree. Pods are 7–12 cm long (), seeds are more than three in a single pod, light or dark brown, kidney-shaped (). E. stricta is distributed across its native range, including India, Bangladesh, Cambodia, China, Laos, Myanmar, Nepal, Thailand, Tibet, Vietnam and the Western Himalayas (POWO, 2023b).
Figure 1. Morphology of Erythrina stricta. A. Habit; B. Flowers; C. Mature pods; D. Seeds; E. Seed oil.
Widely acknowledged in many parts of India for their potent medicinal applications, E. stricta leaves are employed to alleviate joint pains, earache, toothache and eye infections (Umamaheswari et al., Citation2009). The bark is beneficial in treating asthma, epilepsy, rheumatism, itch, stomachache and dysentery. The bark paste is also applied externally to cure eczema, dermatitis and other skin diseases (Umamaheswari et al., Citation2009; Kichu et al., Citation2015; Akter et al., Citation2016). However, the nutritional, phytochemical composition and biological activities of E. stricta remain unrecognized. Thus, this study reports the proximate and mineral composition of E. stricta seeds. We also provide insights into seed oil’s physicochemical properties, fatty acid composition and antinutritional components such as oxalate and phytate. E. stricta stands out as an underutilized legume in India, holding potential for exploration due to its medicinal and nutritional benefits. This pioneering study reveals the noteworthy nutritional and phytochemical composition of E. stricta seeds. Additionally, the research delves into the seed oil’s fatty acid profiling and physicochemical characterization.
2. Materials and methods
2.1. Plant materials and chemicals
The pods of E. stricta were collected from the trees grown near Shiggavi, Haveri district, Karnataka, India (15.010372N, 75.129678E) in March 2022. The seeds () were separated from the pods and dried to make them moisture-free in an oven at 40 ± 2 °C. Dried seeds were powdered using a mechanical grinder and stored in air-tight polythene bags at room temperature until further use. Chemicals, such as Folin-Cicalteau reagent, BF3-methanol, anthrone and standard chemicals, such as bovine serum albumin, glucose and sodium phytate used in this study were procured from Himedia laboratories, Mumbai, India, whereas heptadecanoic acid was purchased from Sigma-Aldrich, Bengaluru. All the other chemicals and solvents used were of analytical grade.
2.2. Proximate analysis
The seeds’ moisture, fat, ash and protein contents were analysed as mentioned in AOAC (Citation2000). Briefly, the moisture content was gravimetrically determined by recording the weight difference of oven-dried sample at 102 °C for 6 h; the oil content () of the sample was obtained gravimetrically – to detail, finely ground powder of seeds was extracted with petroleum ether (40–60 °C) in a Soxhlet apparatus at 65 ± 2 °C for 8 h to get the oil and the solvent fraction was evaporated using a rotary evaporator (Buchi, Rotavapor R-100, Flawil, Switzerland). The oil was kept in an oven at a temperature of 40 ± 2 °C to remove the traces of the solvents until the weight become constant. Further, the oil content was determined gravimetrically and stored at − 20 °C until further analysis; ash content of the samples was determined by igniting the oven-dried samples in the muffle furnace at 750 °C. For protein content analysis, 500 mg of defatted samples were grounded with 5–10 mL of buffer. The known volume of sample extract was taken and made to 1 mL with the distilled water, to which 4.5 mL of reagent C (It is a mixture of 50 mL solution of 2% sodium carbonate in 0.1 N sodium hydroxide and 1 mL of 0.5% copper sulphate (CuSO4.5H2O) in 1% potassium sodium tartarate) was added. After the incubation period of 10 min, 0.5 mL of reagent D (Folin-Cicalteau reagent) was added, and tubes were allowed to stand in the dark at room temperature for 30 min to develop blue colour. The colour developed was measured at 660 nm spectroscopically. Bovine serum albumin was used as a standard (Sadashivam & Manickam, Citation2008). The carbohydrate was quantified by anthrone reagent method and fibre content was by digesting samples with acid and alkali (Sadashivam & Manickam, Citation2008). The energy value was calculated using Atwater-specific conversion factors, as FAO (Citation2003) mentioned.
2.3. Mineral composition analysis
NOVA 400 atomic absorption spectrophotometer (model Analytic Jena, Germany) with an air or acetylene flame was used for the analysis of potassium, phosphorous, sulphur, sodium, calcium, boron, manganese, magnesium, copper, iron and zinc and the absorbance was carried using hollow cathode lamps (AOAC, Citation2000; Fernandez-Hernandez et al., Citation2010). For the nitrogen estimation, a two-step digestion-UV spectrophotometric method was adopted (Liu et al., Citation2013).
2.4. Seed oil characterization
2.4.1. Physicochemical characterization
The colour and physical state of the extracted oil were observed after 4 hours of incubation at room temperature. The density and refractive index of the oil were determined using a specific gravity bottle and Abbe’s refractometer, respectively. Free fatty acid (FFA) content, peroxide value (PV), iodine value, and unsaponification values were determined as per the methods of AOCS (Citation2003). Lignans and carotenoids were quantified by using spectrophotometric methods as described by Manasa et al. (Citation2021).
2.4.2. Fatty acid profiling
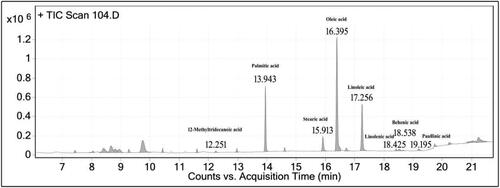
Fatty acid methyl esters (FAME) were synthesized through the esterification process as outlined in AOCS (Citation2003) guidelines. In this procedure, a 15 mg oil sample was combined with 1 mL of BF3-methanol and incubated at 60 °C for 30 min. The reaction tubes were promptly transferred to an ice bath and left there for 5 min. Subsequently, 1 mL each of hexane and distilled water was introduced, and the solution was vortexed. The resulting top layer, consisting of undisturbed methyl esters, was then transferred to GC vials. Heptadecanoic acid served as the internal standard. The identification of FAME was accomplished as described by Manasa et al. (Citation2021) using GC-MS (PerkinElmer, Turbo-mass Gold, Mass spectrometer), equipped with a flame ionization detector (FID) and a fused silica Rtx-2330 column (Restek made, 30 m, 90.32 mm ID and 0.20 mm film thickness). The injector port was maintained at 230 °C, the detector temperature was set at 250 °C, and N2 was employed as the carrier gas. The initial column temperature was 120 °C, gradually increased to 220 °C over 20 min, and held at 220 °C for an additional 10 min. Detection of FAME involved comparing the fragmentation pattern and retention time with established standards and the NIST library.
2.5. Antinutritional factors analysis
2.5.1. Phytate
The defatted seed cake (0.5 g) was extracted with 10 mL of 2.4% HCl for 16 h with constant agitation, and the mixture was filtered. The filtrate was added with 1 g NaCl and constantly shaken for 20 min. The mixture was centrifuged at 1000 g for 20 min at 10 °C, and the known volume of the supernatant obtained was diluted to 3 mL using distilled water, followed by the addition of Wade’s reagent (0.03% FeCl3·6H2O + 0.3% sulfosalicylic acid). The absorbance of the colour developed was read at 500 nm in a UV–Vis spectrophotometer. A control was prepared without the addition of a sample. Sodium phytate was used as a standard (Gao et al., Citation2007).
2.5.2. Oxalate
Oxalate was quantified as per the method of Dye (Citation1956). Defatted seed cake (2 g) was added to 190 mL distilled water and 10 mL of 6 N HCl and heated in a water bath at 90 °C for 4 h. Mixture was filtered, made the volume up to 250 and 50 mL of this solution was titrated against concentrated ammonia in presence of methyl orange indicator and heated to 95 °C followed by the addition of 10 mL of 5% CaCl2. After 10 min, 6 N NH4OH was added and the colour change was observed and kept overnight for the calcium oxalate precipitation. The precipitate was filtered and dissolved in hot sulphuric acid, filtrate was made up to 125 mL, heated to 95 °C, and titrated against 0.05 N KMnO4. Oxalate was determined using following equation;
2.6. Statistical analysis
Each experiment was repeated three times and results are expressed as mean values with standard error. Descriptive statistics including mean and standard error were calculated using Microsoft Excel 2019.
3. Results and discussion
3.1. Proximate and mineral composition
The proximate analysis estimates major nutrient components, including energy value. The proximate composition of seeds of E. stricta is found to be impressive, with a good amount of protein, fat and fibre, as presented in . The seed holds a protein content of 26.81%, fat of 13.43%, fibre of 18.71%, and carbohydrate of 19.34% with 284.15 Kcal/100 g energy value. The moisture and ash content were 15.39% and 6.04%, respectively. The proximate composition of E. stricta is comparable to that of two Mexican species, E. americana and E. breviflora (Sotelo et al., Citation1993). The protein, fat and fibre content of E. americana was 27.2%, 17.7% and 15.3%, respectively, whereas that of E. breviflora was 23.3%, 13.9%, and 21.6%, respectively. However, E. stricta is richer in ash content than E. americana (3.6%) and E. breviflora (3.4%), a direct indicator of minerals. Further, E. stricta accommodates a higher amount of protein, fat, fibre, and ash than some well-known pulses, such as Cicer arietinum, Phaseolus mungo, Pisum sativum and Cajanus cajan (Longvah et al., Citation2017).
Table 1. Proximate and mineral composition of Erythrina stricta seeds.
Minerals play a crucial role in supporting the fundamental physiological functions of the human body, including the development of bones, muscle and nerve functioning, as well as the regulation of water balance (Weyh et al., Citation2022). Legumes are renowned for their mineral-rich composition in seeds; the same holds true for E. stricta (). Specifically, E. stricta exhibits high nitrogen, potassium, phosphorous, magnesium and calcium levels, with concentrations of 16.60, 14.0, 6.47, 6.0 and 5.0 mg/g DW, respectively. Additionally, microelements, such as iron, zinc, manganese, boron and copper present at noteworthy levels, measuring 787.0, 497.0, 55.2, 36.8 and 32.7 µg/g DW, respectively. The mineral content of E. stricta seed is higher than that of E. indica, an allied species, which had 2.35 mg/g calcium, 3.04 mg/g magnesium, 65.7 µg/g iron and 8.1 µg/g copper (Pugalenthi et al., Citation2004). Comparatively, the mineral content of E. stricta seeds surpasses that of commonly consumed pulses such as Cicer arietinum, Phaseolus mungo and Dolichos biflorus. For instance, the calcium content in C. arietinum, P. mungo and D. biflorus is recorded at 1.50, 0.86 and 2.69 mg/g, respectively, while the magnesium content stands at 1.6, 1.9 and 1.52 mg/g, respectively (Longvah et al., Citation2017). Notably, the iron and zinc content of E. stricta seeds significantly surpass those of C. arietinum, P. mungo and D. biflorus, with values of 67.8, 59.7 and 87.6 µg/g iron, and 33.7, 30.5 and 27.1 µg/g zinc, respectively (Longvah et al., Citation2017).
3.2. Seed oil characterization
Some legumes store impressive amounts of oil, besides the rich protein content, with some crops such as Arachis hypogaea (ground nut), Glycine max (soybean) and Pongamia pinnata being grown for the production of seed oil commercially. E. stricta holds a significant oil content () in seeds (13.43%) which is rich in carotenoids and unsaturated fatty acids (USFAs). The physicochemical properties and fatty acid composition of the seed oil are presented in and GC-MS chromatogram of the fatty acid profiling is presented in . The seed oil was liquid and greenish-yellow coloured at room temperature. The refractive index is linked to the molecular weight, degree of unsaturation and length of a fatty acid chain of the oil, and E. stricta had a refractive index of 1.471 which is comparable to that of two common edible oils; soybean oil (1.473) and coconut oil (1.448) (Pantzaris & Basiron, Citation2002; Wang, Citation2002). The density of E. stricta seed oil is 0.910 g/cm3 and comparable to that of coconut oil (0.914 g/cm3); (Pantzaris & Basiron, Citation2002). FFA and PV are essential quality parameters that give a quick impression of their edibility. The FFA content of crude oils would be more compared to that of refined ones, and seed oil with an FFA content of less than 5% could be used for edible purposes (Lamani et al., Citation2021); the FFA of E. stricta was 1.41% and is comparable to that of some commercial edible oils, such as sesame and coconut oil and even less than the mustard (1.9%) and rice bran oil (1.4%) (Prashanth Kumar et al., Citation2017). The PV of E. stricta was 19.91 meq O2/kg, which is slightly more than that of some commercial edible oils, such as mustard, sesame, peanut, and olive oils (Prashanth Kumar et al., Citation2017) and much less compared to the unrefined oil of B. roxburghii (69.98 meq O2/kg), an underutilized species (Yadav et al., Citation2022). The IV (iodine value) indicates the unsaturation level of oil, and it was 79.99 I2/100 g and is comparable to that of two crucial edible refined oils, olive oil (79.5 I2/100 g) and mustard oil (66.0 I2/100 g) (Prashanth Kumar et al., Citation2017). The unsaponification value of E. stricta seed oil was 0.16%, representing the nutraceuticals present in the oil other than the fatty acids. This value is comparable to refined coconut and palm oil, which had values of 0.13% and 0.28%, respectively (Prashanth Kumar et al., Citation2017). Carotenoids are tetraterpenoid pigment molecules that stabilize oil against oxidation and have a beneficial role in eye-related problems (Franke et al., Citation2010). The E. stricta seed oil is rich in carotenoids with a value of 36.18 mg/kg oil, and it is much higher than the earlier report of rapeseed, sunflower, and flax seed oil, which had values of 15.2, 1.6 and 3.7 mg/kg oil, respectively (Franke et al., Citation2010). Lignans are a group of phenolic compounds that are proven to prevent cardiovascular diseases, cancer and cellular oxidative damage, and their presence gives an oil a dietary value (Korhonen, Citation2002). The total lignan content of the studied oil was 165.47 mg/100 g sesamol equivalent (SE), and it is relatively lower than that of sesame oils (495.9–685.6 mg/100 g SE), which is a rich source of dietary lignans (Bhatnagar et al., Citation2015).
Table 2. Physicochemical properties and fatty acid profile of Erythrina stricta seed oil.
The fatty acid composition of E. stricta was impressive, with a significant portion represented by USFAs. Oleic acid was the prominent fatty acid with a presence of 48.82%, followed by palmitic acid (20.63%), linoleic acid (20.27%) and stearic acid (6.47%). Behenic acid, linolenic acid, paullinic acid and 12-methyltridecanoic acid were in minor quantities. The E. stricta seed oil is rich in USFA, which makes up 70.90% of the oil. Matthäus (Citation2007) argues that oils with high monounsaturated fatty acid (MUFA) are more stable to oxidative degradation and are preferable for food frying purposes, and E. stricta seed oil could be the best choice for frying purposes as it accommodates 49.82% of MUFA. A similar pattern in the fatty acid composition of E. variegata (Samanta & Laskar, Citation2013) and E. suberosa (Singh & Chawla, Citation1970) was also reported. The palmitic, stearic, oleic and linoleic acid content of presently studied oil is comparable to that of palm oil, with values of 39.1%, 4.1%, 42.4% and 10.1%, respectively (Matthäus, Citation2007). Seed oils are the primary provider of essential fatty acids, such as α-linolenic acid and linoleic acid that are vital for various biological functions (Yadav et al., Citation2022). The linoleic and α-linolenic acid content of presently studied oil is more when compared to that of some well-known oils, such as olive, rapeseed, palm and almond oil (Kaur et al., Citation2014). Considering these facts, the seed oil of E. stricta, an underutilized legume, could be explored as a new edible oil source in India.
3.3. Antinutritional components
Antinutritional factors reduce the nutrients’ bioavailability and make a food material inefficient. Among the various antinutrients, phytate and oxalate are considered paramount as they bind with minerals and narrow their availability (Samtiya et al., Citation2020). The phytate and oxalate content of E. stricta seeds was 26.85 and 16.04 mg/g FW, respectively (). The phytate is the molecule that plants use to store phosphorus content in seeds, and if consumed regularly, it is associated with iron deficiency (Samtiya et al., Citation2020). The phytate content of E. stricta is similar to that of some well-known pulses, such as bean (18.74 mg/100 g), fava bean (22.85 mg/100 g) and soybean (22.91 mg/100 g) and wild edible species such as Diospyros chloroxylon (20.16 mg/g) and Balanites roxburghii (21.71 mg/g) (Samtiya et al., Citation2020; Yadav et al., Citation2022; Murthy et al., Citation2022). The oxalate content of E. stricta is comparatively higher than that of the pulses mentioned above and comparable to that of wild edible species mentioned above, B. roxburghii (32.01 mg/g) and Diospyros chloroxylon (14.33 mg/g) (Yadav et al., Citation2022; Murthy et al., Citation2022). However, the adoption of some simple processing methods was proved very effective in reducing them. Shi et al. (Citation2018) able to reduce up to 51.89% and 56.29% of soluble and total oxalates, respectively, and phytate to some extent, by soaking the seeds in distilled water for 4 h. Thus, E. stricta seed cake, with impressive mineral composition and other nutraceuticals, could be considered a micronutrient source for humans and cattle.
Table 3. Anti-nutritional factors of Erythrina stricta seeds.
5. Conclusions
The investigation into Erythrina stricta, an underutilized legume species prevalent in the Indian sub-continent, focuses on unraveling its nutritional value. This exploration unveils the potential of E. stricta as a valuable food source. The proximate composition of E. stricta seeds aligns closely with globally recognized pulses, boasting notably high levels of proteins and fibres. Moreover, the seeds are a noteworthy reservoir of oil, enriched with carotenoids and USFAs, particularly the essential linoleic acid. The seed residue exhibits substantial concentrations of essential minerals, including calcium, iron, copper, boron and zinc. This study, thus, endeavours to elucidate the nutritional and oil characterization of E. stricta seeds, positioning them as a promising candidate for novel food sources.
Author contributions
Conceptualization, HNM and GGY.; methodology, HNM, GGY, SSK and SL; software, ASD and MMS; formal analysis, HNM, GGY, SSK, MMS, ASD and SL; investigation, HNM, GGY, SSK, SL, MMS and ASD.; resources, HNM; data curation, HNM, GGY, SSK, SL and MMS.; writing – original draft preparation, HNM and GGY; writing – review and editing, HNM, YHD and KMT; validation, ASD, YHD and KMT; visualization, YHD and KMT. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors are thankful to the University Scientific and Instruments Centre (USIC), Karnatak University, Dharwad, for giving the instrument facility. The authors acknowledge the Researchers Supporting Project number (RSP-2024R375), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
The authors declare no conflicts of interest.
Data availability statement
Data and materials supporting the results or analyses presented in our paper are available upon reasonable request.
Additional information
Funding
Notes on contributors
Hosakatte Niranjana Murthy
Hosakatte Niranjana Murthy was a Professor at the Department of Botany, Karnatak University, India, currently working as a Professor at the Department of Biotechnology, KLE Technological University, Hubballi, India, and Brain Pool Fellow at the Department of Horticulture, Chungbuk National University, Cheongju, Republic of Korea. He is a researcher in the field of Plant Sciences and is involved in teaching graduate and master students.
Guggalada Govardhana Yadav
Guggalada Govardhana Yadav is a Research Scholar at the Department of Botany, Karnatak University, India.
Sathish Shekhappa Kadapatti
Sathish Shekhappa Kadapatti is a Research Scholar at the Department of Botany, Karnatak University, India.
Shrinivas Lamani
Shrinivas Lamani is a Research Scholar at the Department of Botany, Karnatak University, India.
Anita S. Desai
Anita S. Desai is a Master’s student at the Department of Botany, Karnatak University, India.
Megha M. Sumbad
Megha M. Sumbad is a Master’s student at the Department of Botany, Karnatak University, India.
Yaser Hassan Dewir
Yaser Hassan Dewir is a professor at King Saud Univ., Saudi Arabia and Kafrelsheikh Univ., Egypt. He received his MSc from South China Agricultural Univ. (SCAU) China and Ph.D. from Chungbuk National Univ. (CBNU), Korea. He carried out Postdoc. studies in CBNU, Korea and University of KwaZulu-Natal (UKZN), South Africa. His research interests including cell and tissue culture, plant biology and biotechnology.
Katalin Magyar-Tábori
Katalin Magyar-Tábori is a professor at Research Institute of Nyíregyháza, Institutes for Agricultural Research and Educational Farm (IAREF), University of Debrecen, Nyiregyháza, Hungary.
References
- Akter, K., Barnes, E. C., Loa-Kum-Cheung, W. L., Yin, P., Kichu, M., Brophy, J. J., Barrow, R. A., Imchen, I., Vemulpad, S. R., & Jamie, J. F. (2016). Antimicrobial and antioxidant activity and chemical characterisation of Erythrina stricta Roxb. (Fabaceae). Journal of Ethnopharmacology, 185, 1–9. https://doi.org/10.1016/j.jep.2016.03.011
- AOAC. (2000). Official methods of analysis of the association of analytical chemists (17th ed.). Association of Official Analytical Chemists, Inc.
- AOCS. (2003). Official methods and recommended practices of the American Oil Chemist’s Society. American Oil Chemist’s Society.
- Ayilara, M. S., Abberton, M., Oyatomi, O. A., Odeyemi, O., & Babalola, O. O. (2022). Potentials of underutilized legumes in food security. Frontiers in Soil Science, 2, 1020193. https://doi.org/10.3389/fsoil.2022.1020193
- Bhatnagar, A. S., Hemavathy, J., & Gopala Krishna, A. G. (2015). Development of a rapid method for determination of lignans content in sesame oil. Journal of Food Science and Technology, 52(1), 521–527. https://doi.org/10.1007/s13197-013-1012-0
- Dye, W. B. (1956). Chemical studies on Halogeton glomeratus. Weeds, 4(1), 55–60. https://doi.org/10.2307/4040009
- Fahmy, N. M., Al-Sayed, E., El-Shazly, M., & Nasser Singab, A. (2020). Alkaloids of genus Erythrina: An updated review. Natural Product Research, 34(13), 1891–1912. https://doi.org/10.1080/14786419.2018.1564300
- FAO. (2003). Food energy - methods of analysis and conversion factors. FAO.
- Fernandez-Hernandez, A., Mateos, R., Garcia-Mesa, J. A., Beltran, G., & Fernandez-Escobar, R. (2010). Determination of mineral elements in fresh olive fruits by flame atomic spectrometry. Spanish Journal of Agricultural Research, 8(4), 1183–1190. https://doi.org/10.5424/sjar/2010084-1206
- Franke, S., Fröhlich, K., Werner, S., Böhm, V., & Schöne, F. (2010). Analysis of carotenoids and vitamin E in selected oilseeds, press cakes and oils. European Journal of Lipid Science and Technology, 112(10), 1122–1129. https://doi.org/10.1002/ejlt.200900251
- Gao, Y., Shang, C., Maroof, M. A. S., Biyashev, R. M., Grabau, E. A., Kwanyuen, P., Burton, J. W., & Buss, G. R. (2007). A modified colorimetric method for phytic acid analysis in soybean. Crop Science, 47(5), 1797–1803. https://doi.org/10.2135/cropsci2007.03.0122
- Kaur, N., Chugh, V., & Gupta, A. K. (2014). Essential fatty acids as functional components of foods- a review. Journal of Food Science and Technology, 51(10), 2289–2303. https://doi.org/10.1007/s13197-012-0677-0
- Kichu, M., Malewska, T., Akter, K., Imchen, I., Harrington, D., Kohen, J., Vemulpad, S. R., & Jamie, J. F. (2015). An ethnobotanical study of medicinal plants of Chungtia village, Nagaland, India. Journal of Ethnopharmacology, 166, 5–17. https://doi.org/10.1016/j.jep.2015.02.053
- Korhonen, H. (2002). Technology options for new nutritional concepts. International Journal of Dairy Technology, 55(2), 79–88. https://doi.org/10.1046/j.1471-0307.2002.00050.x
- Lamani, S., Anu-Appaiah, K. A., Murthy, H. N., Dewir, Y. H., & Rihan, H. Z. (2021). Fatty acid profile, tocopherol content of seed oil, and nutritional analysis of seed cake of wood apple (Limonia acidissima L.), an underutilized fruit-yielding tree species. Horticulturae, 7(9), 275. https://doi.org/10.3390/horticulturae7090275
- Lim, T. K. (2014). Erythrina variegata. Edible medicinal and non-medicinal plants (pp 788–805). Springer Netherlands. https://doi.org/10.1007/978-94-007-7395-0_63
- Liu, W. J., Zeng, F. X., & Jiang, H. (2013). Determination of total nitrogen in solid samples by two-step digestion–ultraviolet spectrophotometry method. Communications in Soil Science and Plant Analysis, 44(6), 1080–1091. https://doi.org/10.1080/00103624.2012.750330
- Longvah, T., Ananthan, R., Bhaskarachary, K., & Venkaiah, K. (2017). Indian food composition table. ICMR-National Institute of Nutrition.
- Manasa, V., Vaishnav, S. R., & Tumaney, A. W. (2021). Physicochemical characterization and nutraceutical compounds of the selected spice fixed oils. Journal of Food Science and Technology, 58(8), 3094–3105. https://doi.org/10.1007/s13197-020-04813-8
- Matthäus, B. (2007). Use of palm oil for frying in comparison with other high-stability oils. European Journal of Lipid Science and Technology, 109(4), 400–409. https://doi.org/10.1002/ejlt.200600294
- Murthy, H. N., Dalawai, D., Arer, I., Karadakatti, P., & Hafiz, K. (2022). Nutritional value of underutilized fruit: Diospyros chloroxylon Roxb. (green ebony persimmon). International Journal of Fruit Science, 22(1), 249–263. https://doi.org/10.1080/15538362.2021.2023065
- Murthy, H. N., & Paek, K. Y. (2021). Health benefits of underutilized vegetables and legumes. In H. N. Murthy, & K.Y. Paek (Eds.), Bioactive compounds in underutilized vegetables and legumes. Reference Series in Phytochemistry (pp 1–36). Springer. https://doi.org/10.1007/978-3-030-57415-4_1
- Pantzaris, T. P., & Basiron, Y. (2002). The lauric (coconut and palmkernel) oils. In F. D. Gunstone (Ed.), Vegetable oil in food technology; composition, properties and uses (1st ed., pp. 157–202). CRC Press LLC.
- Paterson, R. T. (1994). Use of trees by livestock 9: Erythrina. Natural Resources Institute.
- POWO. (2023a). Erythrina L. https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:30007957-2. Accessed on 24 Dec 2023.
- POWO. (2023b). Erythrina stricta Roxb. https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:494596-1. Accessed on 24 Dec 2023.
- Prashanth Kumar, P. K., Manasa, V., Matthaus, B., Vijayaraj, P., Gopala Krishna, A. G., & Rajashekharan, R. (2017). Study on minor components in some Indian commercial vegetable oils. Beverage and Food World, 44, 21–27. http://ir.cftri.res.in/id/eprint/13231
- Pugalenthi, M., Vadivel, V., Gurumoorthi, P., & Janardhanan, K. (2004). Comparative nutritional evaluation of little known legumes, Tamarindus indica, Erythrina indica and Sesbania bispinosa. Tropical and Subtropical Agroecosystems, 4, 107–123. http://www.redalyc.org/ariculo.oa?id=93940302
- Rambo, D. F., Biegelmeyer, R., Toson, N. S. B., Dresch, R. R., Moreno, P. R. H., & Henriques, A. T. (2019). The genus Erythrina L.: A review on its alkaloids, preclinical, and clinical studies. Phytotherapy Research, 33(5), 1258–1276. https://doi.org/10.1002/ptr.6321
- Sadashivam, S., & Manickam, A. (2008). Biochemical methods (3rd ed.), New Age International (P) Limited, Publishers.
- Samanta, T. D., & Laskar, S. (2013). Analysis of oil and fatty acids from the seeds of Erythrina variegata Linn. Biosciences Biotechnology Research Asia, 10(1), 433–437. https://doi.org/10.13005/bbra/1149
- Samtiya, M., Aluko, R. E., & Dhewa, T. (2020). Plant food anti-nutritional factors and their reduction strategies: An overview. Food Production, Processing and Nutrition, 2(1), 6. https://doi.org/10.1186/s43014-020-0020-5
- Shi, L., Arntfield, S. D., & Nickerson, M. (2018). Changes in levels of phytic acid, lectins and oxalates during soaking and cooking of Canadian pulses. Food Research International (Ottawa, Ont.), 107, 660–668. https://doi.org/10.1016/j.foodres.2018.02.056
- Singh, H., & Chawla, A. S. (1970). Erythrina sp. III: Chemical constituents of Erythrina suberosa Roxb. seeds. Journal of Pharmaceutical Sciences, 59(8), 1179–1182. https://doi.org/10.1002/jps.2600590828
- Sotelo, A., Soto, M., Lucas, B., & Giral, F. (1993). Comparative studies of the alkaloidal composition of two Mexican Erythrina species and nutritive value of the detoxified seeds. Journal of Agricultural and Food Chemistry, 41(12), 2340–2343. https://doi.org/10.1021/jf00036a023
- Umamaheswari, M., Asokkumar, K., Sivashanmugam, A. T., Remyaraju, A., Subhadradevi, V., & Ravi, T. K. (2009). In vitro xanthine oxidase inhibitory activity of the fractions of Erythrina stricta Roxb. Journal of Ethnopharmacology, 124(3), 646–648. https://doi.org/10.1016/j.jep.2009.05.018
- Vilcanqui-Pérez, F., Chaquilla-Quilca, G., Sarmiento-Casavilca, V. H., Céspedes-Orosco, C. N., & VentURa-Saldivar, Y. (2022). Nutritional, physical and sensory characteristics of bread with the inclusion of germinated basul (Erythrina edulis) flour. Journal of Food Science and Technology, 59(6), 2117–2126. https://doi.org/10.1007/s13197-021-05246-7
- Wang, T. (2002). Soybean oil. In F. D. Gunstone (Ed.), Vegetable oil in food technology; composition, properties and uses (1st ed., pp 18–58). CRC Press LLC.
- Weyh, C., Krüger, K., Peeling, P., & Castell, L. (2022). The role of minerals in the optimal functioning of the immune system. Nutrients, 14(3), 644. https://doi.org/10.3390/nu14030644
- Yadav, G. G., Murthy, H. N., & Dewir, Y. H. (2022). Nutritional composition and in vitro antioxidant activities of seed kernel and seed oil of Balanites roxburghii: An underutilized species. Horticulturae, 8(9), 798. https://doi.org/10.3390/horticulturae8090798