Abstract
Trichoderma has been used as a biological control agent against grey mould in major crops because of its large variety of antagonistic mechanisms. In the present study, Trichoderma sp. SANA20 was isolated from Japanese apricot bark as a biological control agent for grey mould caused by Botrytis cinerea. SANA20 suppressed B. cinerea hyphal growth but did not show mycoparasitism on media using the dual culture technique. SANA20 decreased B. cinerea induced grey mould disease severity in cucumber leaves. The biocontrol activity of SANA20 culture media toward B. cinerea induced grey mould disease suggested that the antagonistic activity of SANA20 toward B. cinerea was dependent on substances secreted by SANA20. SANA20 produced and secreted chitinase, which can degrade fungal cell wall, in culture media. Purified chitinase from SANA20 culture media also decreased the severity of grey mould in cucumber leaves, suggesting that chitinase production by SANA20 played a critical role in the biological control of B. cinerea. SANA20 is expected to contribute to the further improvement of integrated pest management strategies for combating B. cinerea and to potentially suppress the heavy use of chemical fungicides in the field.
PUBLIC INTEREST STATEMENT
Trichoderma sp. SANA20 was isolated from Japanese apricot bark as a biological control agent for grey mould caused by Botrytis cinerea. Chitinase production by SANA20 played a critical role in the biological control of B. cinerea. SANA20 is expected to contribute to the further improvement of integrated pest management strategies for combating B. cinerea and to potentially suppress the heavy use of chemical fungicides in the field.
Competing Interests
The authors declares no competing interests.
1. Introduction
Grey mould caused by the necrotrophic plant pathogen Botrytis cinerea Pers ex Fr. (anamorph of Botryotinia fuckeliana (de Bary) Whetz) is one of the economically important diseases in agriculture. B. cinerea infects over 200 plant species and causes annual losses of 10 USD billion to 100 USD billion worldwide (Boddy, Citation2016). Fungicide application is a simple strategy to protect crops from B. cinerea attack. However, the emergence of fungicide-resistant isolates from B. cinerea populations has become a serious problem. B. cinerea belongs to plant pathogens showing a high risk of development of resistance to fungicides (FRAC, Citation2019). Such dicarboximides as iprodione and procymidone, which interfere with osmotic signal transduction in B. cinerea (Oshima et al., Citation2002), have superseded benzimidazoles, which inhibit β-tubulin polymerization (Butters & Hoolomon, Citation1999), but dicarboximide-resistant B. cinerea isolates were found in 1977 (Pommer & Lorenz, Citation1987). Fenhexamid, which inhibits 3-ketoreductase in the C4-demethylation enzyme complex (Debieu et al., Citation2001), has been used in many European countries. However, fenhexamid-resistant B. cinerea isolates were detected from field isolates shortly after its introduction (Fillinger et al., Citation2008). Thus, it is difficult to control grey mould with chemical fungicides and farmers face the critical problem of how to control grey mould in the field every year.
Because of the emergence of fungicide-resistant B. cinerea populations as well as environmental concerns brought about chemical fungicide application in the field, the use of biological control agents to control B. cinerea growth has become popular (Elmer & Reglinski, Citation2006). Biological control agents are generally microorganisms. They possess antagonistic effects on plant pathogen growth, including spore germination and hyphal growth, on host plants (Dik et al., Citation1999). Most commercially available biological control agents are Bacillus (Ongena & Jacques, Citation2008). Bacillus produces a large number of antibiotics (Stein, Citation2005). For example, the cyclic lipopeptide, iturin A, inhibited B. cinerea hyphal growth and mutants lacking two genes that play a role in iturin A production lost their antagonistic activity toward B. cinerea (Furuya et al., Citation2011).
Antagonistic fungi have been studied as candidates for biological control agent. Trichoderma is one of the most well-studied fungi as a prominent biological control agent (Elad, Citation2000a). Trichoderma harzianum T39 is a commercial biocontrol agent (TRICHODEX, Makhteshim Chemical Works) that controls B. cinerea disease in greenhouses and vineyards (Elad, Citation2000b). The potential modes of biological control by Trichoderma include mycoparasitism (Elad, Citation1995), competition (Blakeman, Citation1993), antibiotic production (Webster & Lomas, Citation1964), induction of plant resistance (Elad et al., Citation1998), degradation of plant pathogen enzymes by proteases (Elad & Kapat, Citation1999), and degradation of plant pathogen cell wall by degrading enzymes (Lorito et al., Citation1993). Some of these modes cooperatively act in the biological control of plant diseases by Trichoderma (Howell, Citation2007).
We report herein the isolation and characterization of Trichoderma sp. SANA20, which is isolated from the bark of Japanese apricot, as a biological control agent for grey mould caused by B. cinerea.
2. Materials and methods
2.1. Isolation of Trichoderma species from Japanese apricot bark
Bark samples were collected from Japanese apricot (Prunus mume) growing in the botanical research garden of the New Technology Development Foundation on 8 August 2016. Briefly, 21 bark samples (approximately 4 cm square) were peeled off from trunks and put on selective agar media to isolate Trichoderma species (Askew & Laing, Citation1993). The media were incubated at 25 ºC until fungal colony formation. Single colonies growing on the media were picked up using an inoculating loop and inoculated on new selective agar media. By repeating the same procedure, Trichoderma species were isolated.
2.2. Identification of Trichoderma isolates
Trichoderma isolates were incubated on potato sucrose agar (PSA) at 25 ºC for 7 days. Hyphae of the isolates were collected using an inoculating loop and homogenized in a mortar containing liquid nitrogen using a pestle. Genomic DNA was isolated from the pulverized samples with a NucleoSpin Plant II (Takara, Otsu, Japan) in accordance with the manufacturer’s instructions. The PCR reaction mixture consisted of 1 µL of 10 × PCR buffer, 0.8 µL of 2.5 mM dNTPs, 0.25 µL of 20 µM primers, 0.1 µL of Taq Hot Start Version (Takara), 1 µL of DNA solution, and 6.6 µL of H2O. PCR conditions were as follows: after incubation at 94 ºC for 3 min, PCR amplification was performed for 35 cycles at 96 ºC for 10 sec, 56 ºC for 30 sec, and 72 ºC for 30 sec, with a final extension step at 72 ºC for 5 min. The nucleotide sequences of the primer set for amplification of fungal internal transcribed spacers (ITSs) were as follows: ITS-1, 5ʹ-TCCGTAGGTGAACCTGCGG-3ʹ and ITS-4, 5ʹ-TCCTCCGCTTATTGATATGC-3ʹ (White et al., Citation1990). DNA sequences of the PCR products were determined using a dye terminator method and subjected to the BLAST homology search system.
2.3. In vitro bioassay
To screen for antagonistic Trichoderma isolates toward B. cinerea, Trichoderma isolates were tested using the dual culture technique as described previously (Furuya et al., Citation2011). Trichoderma isolates or field-isolated B. cinerea was incubated on plates of PSA or potato dextrose agar (PDA) for 3 days, respectively. Hyphal plugs (7 mm diameter) of Trichoderma isolates and B. cinerea field isolate were placed at both ends of the PSA plates, respectively. Incubation was carried out at 25 ºC for 4 days. The antagonistic activity of each isolate was determined by examining growth inhibition of B. cinerea colony with the naked eye and the morphological alteration of B. cinerea hyphae under a light microscope. In vitro assay was repeatedly performed three times.
2.4. Antagonistic activity of SANA20 toward B. cinerea on cucumber seedlings
Seedlings of cucumber (Cucumis sativus L. cv. SHARP-1) with five to six expanding leaves were cultivated in a growth chamber at 27 ºC under light irradiation (11.8 Wm−2/16 h/d). Trichoderma sp. SANA20, selected by in vitro bioassay, was incubated on a PSA plate at 25 ºC for 7 days. Five milliliters of sterilized water was poured into each plate and spores were removed with a brush. Finally, a spore suspension was prepared at 2 × 107 spores/mL with sterilized water. Field-isolated B. cinerea was cultured on PDA plates at 25 ºC for 3 days. The adaxial surfaces of the first to the third true leaves on three seedlings were pretreated with 1 mL of the spore suspension or sterilized water (control) per leaf using an atomizer. After air-drying for 6 h, the center of each pretreated leaf was punctured with a sterile needle and a B. cinerea hyphal plug (5 mm diameter) was placed there. The seedlings were incubated in a growth chamber at 27 ºC for 7 days under light irradiation (11.8 Wm−2/16 h/day). Disease severity was scored on days 3, 5, and 7 after incubation by evaluating the lesion of each leaf according to the following indices: 0, no infection; 1, disease symptom two times larger than the plug; 2, disease symptom four times larger than the plug; 3, disease symptom more than six times larger than the plug; 4, rot more than eight times larger than the plug.
2.5. Antagonistic activity of SANA20 culture supernatant toward B. cinerea on detached cucumber leaves
SANA20 was incubated on a PSA plate at 25 ºC for 7 days. A hyphal plug was inoculated into a 50 mL flask containing 20 mL of potato sucrose broth (PSB). The flask was shaken at 25 ºC at 100 rpm. On day 7 after incubation, 200 μL of culture medium was collected and filtered through a membrane filter (0.20 μm cellulose acetate, Macherey–Nagel, Düren, Germany). Non-inoculated PSB was incubated under the same conditions, filtered through the membrane filter, and used as control. The second true leaves were detached from the cucumber seedlings. Hyphal plugs of B. cinerea were prepared as mentioned above. The adaxial surfaces of three detached leaves were sprayed with 1 mL of the culture supernatant or non-inoculated PSB (control) using an atomizer. After air-drying for 6 hrs, the center of each leaf was punctured with a sterile needle and a B. cinerea hyphal plug (5 mm diameter) was placed there. The leaves were placed adaxial side up on moistened filter paper in Petri dishes. The dishes were incubated at 25 ºC under light irradiation (11.8 Wm−2/16 h/day). Disease severity was scored as mentioned above.
2.6. Determination of chitinase production by SANA20
SANA20 was incubated on a PSA plate at 25 ºC for 7 days. A hyphal plug was inoculated into a 50 mL flask containing 20 mL of PSB. Five flasks were prepared and shaken at 25 ºC at 100 rpm. On days 0, 1, 3, 5, and 7 after incubation, 200 μL of culture medium was collected and filtered through a membrane filter (0.20 μm cellulose acetate, Macherey–Nagel). Chitinase activity in the SANA20 culture medium was determined by measuring the increase in reducing power of a reaction mixture in accordance with literature (Uchibori et al., Citation2000) with slight modification. Briefly, a reaction mixture consisting of 390 μL of a substrate solution [1.6% β-chitin (Sunfive, Tottori, Japan) in 100 mM sodium acetate buffer, pH 5.0] and 10 μL of the culture medium was incubated at 40 ºC for 5 min. The reaction was stopped by adding 800 μL of 0.5 M potassium ferricyanide. After boiling for 15 min, the reaction mixture was centrifuged at 10,000 g at 4 ºC for 15 min. The absorbance of the supernatant at 420 nm was measured. The amount of reducing groups was calculated on the basis of the calibration curve made with N-acetyl-D-glucosamine. One unit (μmol/min) of enzyme activity was defined by the amount of enzyme that liberated reducing sugar equivalent to 1 μmol of N-acetyl-D-glucosamine from β-chitin per minute under the above conditions.
2.7. Purification of SANA20 chitinase
SANA20 was incubated on a PSA plate at 25 ºC for 7 days. A hyphal plug was inoculated into a 50 mL flask containing 20 mL of PSB. The flask was shaken at 25 ºC for 7 days at 100 rpm. The SANA20 culture medium was filtered through membrane filter (0.20 μm cellulose acetate, Macherey–Nagel). Proteins including chitinase were precipitated from the filtrate by adding ammonium sulfate (80% saturation) as described previously (Saito et al., Citation2011). The precipitate was dissolved in 100 mM potassium phosphate buffer (pH 7.0). After removal of ammonium sulfate by dialysis with 100 mM potassium phosphate buffer (pH 7.0), chitin magnetic beads (New England BioLab, MA) were incubated with the solution and then chitinase was purified in accordance with the manufacturer’s instructions. Enzyme activity of purified chitinase was measured as mentioned above and prepared at 4.0 units/mL with Milli-Q water.
2.8. Antagonistic activity of chitinase produced by SANA20 toward B. cinerea on detached cucumber leaves
The second true leaves were detached from the cucumber seedlings. Hyphal plugs of B. cinerea were prepared as mentioned above. The adaxial surfaces of three detached leaves were sprayed with 1 mL of purified chitinase (4.0 units/mL) or sterilized water (control) using an atomizer. After air-drying for 6 hrs, the center of each leaf was punctured with a sterile needle and a B. cinerea hyphal plug (5 mm diameter) was placed there. The leaves were placed adaxial side up on moistened filter paper in Petri dishes. The dishes were incubated at 25 ºC under light irradiation (11.8 Wm−2/16 h/day). Disease severity was scored as mentioned above.
2.9. Statistics
Data are expressed as means ± standard deviations of biological replicates. The significance of the difference of biological replicates was determined by analysis of variance (ANOVA) and Student’s t-test using Excel statistics software 2012 (Social Survey Research Information, Tokyo, Japan).
3. Results
3.1. Isolation and identification of Trichoderma SANA20
Twenty-one Trichoderma candidates were isolated on Trichoderma-selective agar media from Japanese apricot. By PCR analysis using ITS primers, all candidates belonged to Trichoderma species. All Trichoderma isolates were subjected to the in vitro bioassay. One Trichoderma isolate that exhibited high antagonistic activity toward B. cinerea hyphal growth was isolated and named Trichoderma sp. SANA20. However, SANA20’s species was not determined by ITS sequences in the present study.
Morphologies of SANA20 colony (Figure ) and hyphae (Figure ) on PSA indicated that SANA20 is a Trichoderma species (Bissett, Citation1984; Chaverri & Samuels, Citation2003; Jaklitsch, Citation2009). SANA20 colony on PSA grew rapidly, producing white and green cushions of sporulating filaments (Figure ). SANA20 conidiophores were highly branched (Figure ). Conidiophores produced side branches (arisen at or near 90°) bearing short phialides (Figure ). SANA20 conidium was produced on the tips of the phialides (Figure ). Conidia were smooth and ellipsoidal (approximately 3–5 × 2–4 µm).
Figure 1. Antagonistic activity of SANA20 toward B. cinerea hyphal growth. (a) SANA20 colony on PSA. (b) Hyphae, conidiophores, and conidia of SANA20 on PSA. Bars, 30 μm. (c) Antagonistic activity of SANA20 toward B. cinerea in in vitro bioassay. B. cinerea was co-incubated with SANA20 using the dual culture technique. (d) Morphology of B. cinerea hyphae in in vitro bioassay. Hyphal tips were ruptured by dual culture with SANA20 (top). Morphology of normal B. cinerea hyphae (bottom). Bar, 200 μm
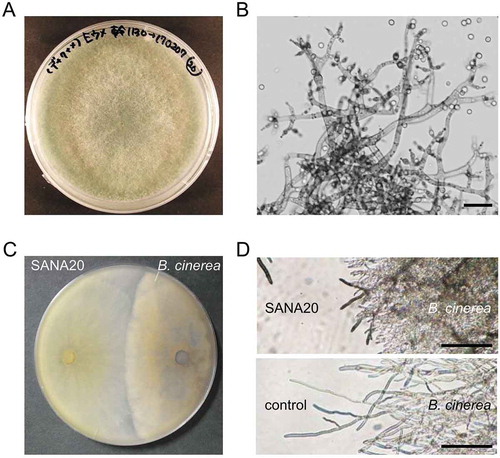
The antagonistic activity of SANA20 toward B. cinerea was detected by the dual culture technique (Figure ). SANA20 inhibited B. cinerea hyphal growth, resulting in narrow inhibition zones between SANA20 and B. cinerea colonies. Hyphal tips of growth-inhibited B. cinerea ruptured without directly contacting SANA20 hyphae (Figure ). In addition, no hook-shaped contact branches indicating mycoparasitism were observed on SANA20 hyphae (Figure ). These observations suggested that SANA20 produced antifungal products that induced the rupture of B. cinerea hyphae in the culture media.
3.2. Antagonistic activity of SANA20 toward B. cinerea
SANA20 significantly reduced B. cinerea induced disease severity in cucumber seedlings (Figure ). Compared with the symptoms in control seedlings, the symptoms caused by B. cinerea infection were less visible by SANA20 treatment on days 3, 5, and 7 after incubation (Figure ). Disease severity in SANA20-treated leaves (Figure )), calculated on the basis of the disease severity index (Figure ), was 0.33, 1.00, and 1.00 on days 3, 5, and 7 after incubation, respectively, and was significantly lower than those of control (2.00, 2.67, and 3.67 on days 3, 5, and 7 after incubation, respectively).
Figure 2. Antagonistic activity of SANA20 toward B. cinerea on cucumber seedlings. (a) Representative symptoms of B. cinerea on leaves treated with SANA20 or sterilized water (control) on days 3, 5, and 7 after incubation. (b) Disease severity index. (c) Disease severity. Bars indicate means ± standard deviations of triplicate experiments (n = 9). Mean values that are statistically different from control (P < 0.01) are indicated by an asterisk
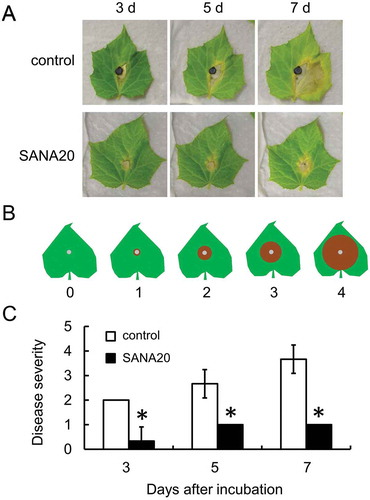
Taken together, the results suggest that SANA20 is able to suppress B. cinerea growth and infection on cucumber.
3.3. Antagonistic activity of SANA20 culture supernatant toward B. cinerea
SANA20 culture supernatant significantly reduced B. cinerea induced disease severity in cucumber leaves (Figure ). Compared with the symptoms on control leaves, the symptoms caused by B. cinerea infection were less visible as a result of treatment with SANA20 culture supernatant on days 3, 5, and 7 after incubation (Figure ). Disease severity in the culture supernatant-treated leaves (Figure ), calculated on the basis of the disease severity index (Figure ), was less than 1.0 (0.30, 0.70, and 0.70 on days 3, 5, and 7 after incubation, respectively) and significantly lower than those of control (2.00, 3.00, and 4.00 on days 3, 5, and 7 after incubation, respectively). The results suggest that SANA20 produces and secretes substances having an antagonistic activity toward B. cinerea.
Figure 3. Antagonistic activity of SANA20 culture supernatant toward B. cinerea on detached leaves of cucumber. (a) Representative symptoms of B. cinerea on leaves treated with SANA20 culture supernatant or non-inoculated PSB (control) on days 3, 5, and 7 after incubation. (b) Disease severity. Bars indicate means ± standard deviations of triplicate experiments (n = 9). Mean values that are statistically different from control (P < 0.01) are indicated by an asterisk
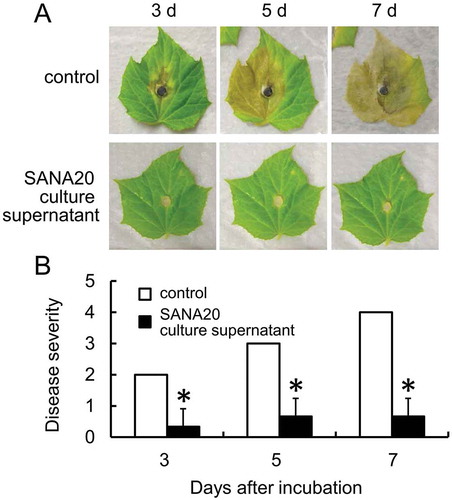
3.4. Antagonistic activity of chitinase produced by SANA20 toward B. cinerea
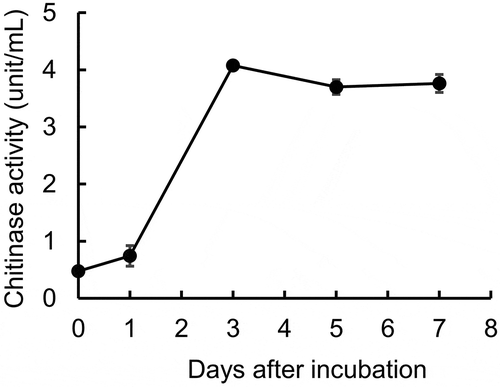
To determine whether chitinase activity was detected in SANA20 culture supernatant, culture media on days 0, 1, 3, 5, and 7 after incubation were subjected to measurement of chitinase activity. SANA20 produced and secreted chitinase in the culture media (Figure ). Peak chitinase activity of approximately 4.0 units/mL in culture media was observed on day 3 after incubation and the activity plateaued thereafter.
Figure 4. Chitinase production by SANA20. Bars indicate means ± standard deviations of five culture media at the indicated incubation periods (n = 5)
Chitinase produced by SANA20 inhibited B. cinerea hyphal growth in vitro. An inhibition zone was formed around a hole filled with chitinase compared with control (Figure ). Microscopic observation showed that the hyphal tips of B. cinerea inhibited by chitinase ruptured compared to the morphology of control hyphae.
Figure 5. Antagonistic activity of chitinase produced by SANA20 toward B. cinerea on detached leaves of cucumber. (a) Inhibition of B. cinerea hyphal growth by chitinase produced by SANA20. Sterilized water was used as a control. Morphological alteration of B. cinerea hyphae was observed under a light microscope. Bar, 200 μm. (b) Representative symptoms of B. cinerea on leaves treated with chitinase produced by SANA20 or sterilized water (control) on days 3, 5, and 7 after incubation. (c) Disease severity. Bars indicate means ± standard deviations of triplicate experiments (n = 9). Mean values that are statistically different from control (P < 0.01) are indicated by an asterisk
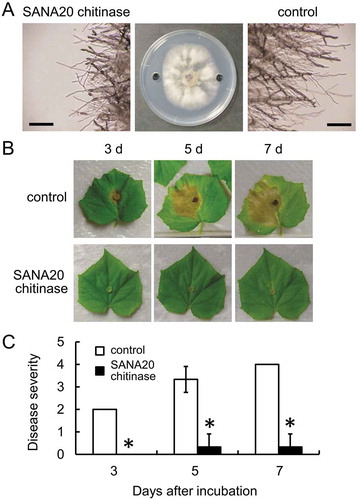
Chitinase produced by SANA20 significantly reduced B. cinerea induced disease severity in cucumber leaves (Figure ). Compared with the symptoms in control leaves, the symptoms resulting from B. cinerea infection were less visible by chitinase treatment on days 3, 5, and 7 after incubation (Figure ). As shown in Figure , disease severity in chitinase-treated leaves, calculated on the basis of the disease severity index (Figure ), was less than 0.5 (0, 0.30, and 0.30 on days 3, 5, and 7 after incubation, respectively) and significantly lower than that of control (2.00, 3.30, and 4.00 on days 3, 5, and 7 after incubation, respectively).
4. Discussion
Because of its large variety of antagonistic mechanisms, Trichoderma has been used as a biological control agent against grey mould in major crops (Talla et al., Citation2015). Trichoderma species produce a variety of exo- and endo-chitinases (Lorito, Citation1988). Trichoderma chitinases can fundamentally suppress B. cinerea growth as well as other plant pathogens (Loc et al., Citation2020). Endo-chitinase produced by T. harzianum inhibited B. cinerea spore germination and germ tube elongation (Lorito et al., Citation1993). The overexpression of Trichoderma chitinase gene suppressed B. cinerea infection in plant (Gentile et al., Citation2007). Trichoderma transformants that overexpressed Trichoderma chitinase gene showed enhanced antagonistic activity toward B. cinerea compared with wild type (Limón et al., Citation2004). On the other hand, the antagonistic mechanism of T. harzianum T39, a commercial biocontrol agent (Elad, Citation2000b), toward B. cinerea is independent of chitinase (Elad & Kapat, Citation1999). Some Trichoderma species are mycoparasites and their chitinases contribute to mycoparasitic attack by loosening the cell walls of host fungi (Seidl-Seiboth et al., Citation2014). The initial step of mycoparasitism between Trichoderma sp. and a fungal prey is hyphal contact (Guzmán-Guzmán et al., Citation2019). In the present study, we could not determine any contact between SANA20 and B. cinerea hyphae under microscopic observation. Therefore, we focused chitinase production by SANA20 in the biological control of B. cinerea growth. Thus, chitinases produced by Trichoderma species may show some redundancy. Through microscopic observation, we confirmed that SANA20 did not form hook-shaped contact branches on B. cinerea hyphae and SANA20 is not parasitic on B. cinerea. In addition, both SANA20 culture supernatant and purified chitinase directly decreased B. cinerea induced disease severity. Hence, one possible mechanism underlying the suppression of B. cinerea hyphal growth by SANA20 could be chitinase production. The generation of genetically modified SANA20 that can secrete a large amount of chitinase on plant surfaces may be our next goal to enhance the antifungal activity of SANA20 toward B. cinerea.
Taken together, SANA20 is a promising biological control agent for grey mould caused by B. cinerea. One of the advantages of biological control agents over chemical fungicides is that they suppress the emergence of fungicide-resistant B. cinerea. At present, microbial fungicides that use Trichoderma species to suppress grey mould disease are not available commercially in Japan. Therefore, SANA20 is expected to contribute to the improvement of integrated pest management strategies for combating B. cinerea and to potentially suppress the heavy use of chemical fungicides in the field. Unfortunately, we did not perform any field test as required by the European and Mediterranean Plant Protection Organization (EPPO) 1999 Guidelines (http://www.eppo.org/). We determined the inhibitory effect of SANA20 on only cucumber grey mould. Environmental conditions frequently influence the inhibitory effects of biological control agents (Benitez et al., Citation2004). Further characterization of SANA20 in various fields and greenhouses based on EPPO guidelines is required to extend the applicability of SANA20 to grey mould in various crops.
Additional information
Funding
Notes on contributors
Shunji Suzuki
Shunji Suzuki is working as a professor at The Institute of Enology and Viticulture, University of Yamanashi in Japan since 2007. He had Bsc, Msc and Ph. D. in Plant Pathology from Mie University in Japan. Then, he worked as a Postdoctoral Researcher at Department of Pharmacology/Toxicology, School of Medicine, University of California, Davis for three years and as an Assistant Professor at Department of Molecular Cell Physiology, School of Medicine, Ehime University for three years. Therefore, his scientific background is based on Immunology as well as Plant Pathology. His current research has focused on plant-microbe interactions and biological control in plant disease control. Also, he has mastered plant breeding techniques to breed molecular modified crops.
References
- Askew, D. J., & Laing, M. D. (1993). An adapted selective medium for the quantitative isolation of Trichoderma species. Plant Pathology, 42(5), 686–11. https://doi.org/10.1111/j.1365-3059.1993.tb01553.x
- Benitez, T., Rincon, A. M., Limon, M. C., & Codon, A. C. (2004). Biocontrol mechanisms of Trichoderma strains. International Microbiology: The Official Journal of the Spanish Society for Microbiology, 7(4), 249–260. https://doi.org/10.2436/im.v7i4.9480
- Bissett, J. (1984). A revision of the genus Trichoderma. I. Section Longibrachiatum sec. nov. Canadian Journal of Botany, 62(5), 924–931. https://doi.org/10.1139/b84-131
- Blakeman, J. P. (1993). Pathogens in the foliar environment. Plant Pathology, 42(4), 479–493. https://doi.org/10.1111/j.1365-3059.1993.tb01528.x
- Boddy, L. (2016). Pathogens of autotrophs. In S. C. Watkinson, N. Money, & L. Boddy (Eds.), The Fungi (pp. 245–292). Academic Press.
- Butters, J. A., & Hoolomon, D. W. (1999). Resistance to benzimidazole can be caused by changes in β-tubulin isoforms. Pesticide Science, 55(4), 486–503. https://doi.org/10.1002/(SICI)1096-9063(199904)55:4<501::AID-PS948>3.0.CO;2-8
- Chaverri, P., & Samuels, G. J. (2003). Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with green ascospores. Studies in Mycology, 48(48), 1–116.
- Debieu, D., Bach, J., Hugon, M., Malosse, C., & Leroux, P. (2001). The hydroxyanilide fenhexamid, a new sterol biosynthesis inhibitor fungicide efficient against the plant pathogenic fungus Botryotinia fukeliana (Botrytis cinerea). Pest Management Science, 57(11), 1060–1067. https://doi.org/10.1002/ps.394
- Dik, A. J., Koning, G., & Köhl, J. (1999). Evaluation of microbial antagonists for biological control of Botrytis cinerea stem infection in cucumber and tomato. European Journal of Plant Pathology, 105(2), 115–122. https://doi.org/10.1023/A:1008623210
- Elad, Y. (1995). Mycoparasitism. In K. Kohmoto, U. S. Singh, & R. P. Singh (Eds.), Pathogenesis and host specificity in plant diseases: Histopathological, biochemical, genetic and molecular basis, eukaryotes (Vol. II, pp. 289–307). Elsevier.
- Elad, Y. (2000a). Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Protection, 19(8–10), 709–714. https://doi.org/10.1016/S0261-2194(00)00094-6
- Elad, Y. (2000b). Trichoderma harzianum T39 preparation for biocontrol of plant diseases-control of Botrytis cinerea, Sclerotinia sclerotiorum and Cladosporium fulvum. Biocontrol Science and Technology, 10(4), 499–507. https://doi.org/10.1080/09583150050115089
- Elad, Y., & Kapat, A. (1999). The role of Trichoderma harzianum protease in the biocontrol of Botrytis cinerea. European Journal of Plant Pathology, 105(2), 177–189. https://doi.org/10.1023/A:1008753629207
- Elad, Y., Kirshner, B., Nitzani, Y., & Sztejnberg, A. (1998). Management of powdery mildew and grey mold of cucumber by Trichoderma harzianum T39 and Ampelomyces quisqualis AQ10. BioControl, 43(2), 241–251. https://doi.org/10.1023/A:1009919417481
- Elmer, P. A. G., & Reglinski, T. (2006). Biosuppression of Botrytis cinerea in grapes. Plant Pathology, 55(2), 155–177. https://doi.org/10.1111/j.1365-3059.2006.01348.x
- Fillinger, S., Leroux, P., Auclair, C., Barreau, C., Hajj, C. A., & Debieu, D. (2008). Genetic analysis of fenhexamid resistant field isolates of the phytopathogenic fungus Botrytis cinerea. Antimicrobial Agents and Chemoterapy, 52(11), 3933–3940. Epub 2008 Sep 8. https://doi.org/10.1128/AAC.00615-08
- FRAC. (2019, October 4). FRAC pathogen list 2019. https://www.frac.info/docs/default-source/publications/pathogen-risk/frac-pathogen-list-2019.pdf
- Furuya, S., Mochizuki, M., Aoki, Y., Kobayashi, H., Takayanagi, T., Shimizu, M., & Suzuki, S. (2011). Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Science and Technology, 21(6), 705–720. https://doi.org/10.1080/09583157.2011.574208
- Gentile, A., Deng, Z., La Malfa, S., Distefano, G., Domina, F., Vitale, A., Polizzi, G., Lorito, M., & Tribulato, E. (2007). Enhanced resistance to Phoma tracheiphila and Botrytis cinerea in transgenic lemon plants expressing a Trichoderma harzianum chitinase gene. Plant Breeding, 126(2), 146–151. https://doi.org/10.1111/j.1439-0523.2007.01297.x
- Guzmán-Guzmán, P., Porras-Troncoso, M. D., Olmedo-Monfil, V., & Herrera-Estrella, A. (2019). Trichoderma species: Versatile plant symbionts. Phytopathology, 109(1), 6–16. https://doi.org/10.1094/PHYTO-07-18-0218-RVW
- Howell, C. R. (2007). Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Disease, 87(1), 4–10. https://doi.org/http://dx.doi.10.1094/PDIS.2003.87.1.4
- Jaklitsch, W. M. (2009). European species of Hypocrea Part I. The green-spored species. Studies in Mycology, 63, 1–93. https://doi.org/10.3114/sim.2009.63.01
- Limón, M. C., Chacón, M. R., Mejías, R., Delgado-Jarana, J., Rincón, A. M., Codón, A. C., & Benítez, T. (2004). Increased antifungal and chitinase specific activities of Trichoderma harzianum CECT 2413 by addition of a cellulose binding domain. Applied Microbiology and Biotechnology, 64(5), 675–685. https://doi.org/10.1007/s00253-003-1538-6
- Loc, N. H., Huy, N. D., Quang, H. T., Lan, T. T., & Ha, T. T. T. (2020). Characterisation and antifungal activity of extracellular chitinase from a biocontrol fungus, Trichoderma asperellum PQ34. Mycology, 11(1), 38–48. https://doi.org/10.1080/21501203.2019.1703839
- Lorito, M. (1988). Chitinolytic enzymes abs their genes. In G. E. Harman & C. P. Kubicek (Eds.), Trichoderma and Gliocladium (pp. 73–99). Taylor and Francis.
- Lorito, M., Harman, G. E., Hayes, C. K., Broadway, R. M., Tronsmo, A., Woo, S. L., & Di Pietro, A. (1993). Chitinolytic ENZYMES Produced by Trichoderma harzianum: Antifungal activity of purified Endochitinase and chitobiosidase. Phytopathology, 83(3), 302–307. https://doi.org/10.1094/Phyto-83-302
- Ongena, M., & Jacques, P. (2008). Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends in Microbiology, 16(3), 115–125. https://doi.org/10.1016/j.tim.2007.12.009
- Oshima, M., Fujimura, M., Banno, S., Hashimoto, C., Motoyama, T., Ichiishi, A., & Yamaguchi, I. (2002). A point mutation in the two-component histidine kinase BcOS1 gene confers dicarboximide resistance in field isolates of Botrytis cinerea. Phytopathology, 92(1), 75–80. https://doi.org/10.1094/PHYTO.2002.92.1.75
- Pommer, E.-H., & Lorenz, G. (1987). Dicarboximide fungicides. In H. Lyr (Ed.), Modern selective fungicides (pp. 99–118). Gustav Fischer.
- Saito, S., Odagiri, M., Furuya, S., Suzuki, S., & Takayanagi, T. (2011). Inhibitory effect of chitinases isolated from Semillon grapes (Vitis vinifera cv.) on growth of grapevine pathogens. Journal of Plant Biochemistry and Biotechnology, 20(1), 47–54. https://doi.org/10.1007/s13562-010-0025-2
- Seidl-Seiboth, V., Ihrmark, K., Druzhinina, I., & Karlsson, M. (2014). Molecular evolution of Trichoderma chitinases. In V. K. Gupta, M. Schmoll, A. Herrera-Estrella, R. S. Upadhyay, I. Druzhinina, & M. G. Tuohy (Eds.), Biotechnology and biology of Trichoderma (pp. 67–78). Elsevier.
- Stein, T. (2005). Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Molecular Microbiology, 56(4), 845–857. https://doi.org/10.1111/j.1365-2958.2005.04587.x
- Talla, S. G., Raju, A. S. R., Karri, S., & Kumar, Y. S. (2015). Production and antagonistic effect of Trichoderma spp. against pathogenic microorganisms (Botrytis cinerea, Fusarium oxysporium, Macrophomina phasealina and Rhizoctonia solani). African Journal of Biotechnology, 14(8), 668–675. https://doi.org/http://dx.doi.10.5897/AJB2014.13904
- Uchibori, T., Yokotsuka, K., & Takayanagi, T. (2000). Purification and characterization of elicitor-induced chitinase from grape berries. Journal of Applied Glycoscience, 47(2), 163–168. https://doi.org/10.5458/jag.47.163
- Webster, J., & Lomas, N. (1964). Does Trichoderma viride produce gliotoxin and viridin? Transactions of the British Mycological Society, 47(4), 535–540. https://doi.org/10.1016/S0007-1536(64)80031-0
- White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press.

