Abstract
Background and Objective:Studies have reported that the spouses of patients with recurrent miscarriage have a high rate of sperm aneuploidy,The types and the methods of determination of sperm aneuploidy are different. The correlation between the specific type of sperm aneuploidy and recurrent miscarriage is inconclusive.Here, we performed a systematic review and meta-analysis to investigate the rate of sperm aneploidy tested in FISH way in male partners of women with recurrent pregnancy loss (RPL group) and fertile male control groups. Methods:Cochrane, Embase, Web of Science, and PubMed databases and a manual search were done for observational research from inception till 23 May 2019.Pooled standard mean difference (SMD), odds ratios (ORs) and 95% confidence interval (CI) were used to describe effect sizes.This study was conducted according to MOOSE statement. Results:Overall, One study was included for qualitative analysis owing to a lack of standard deviation data, and nine studies were included for quantitative analysis.326 male partners of women with recurrent pregnancy loss and 124 fertile men were included in this study.The primary outcome was the rate of sperm aneuploidy. Pooled data from three studies with sufficient data suggested that male partners of women with a history of RPL had significantly higher rates of total sperm aneuploidy compared with the partners of fertile control women(SMD: 1.07, 95% CI: 0.39–1.75, P < 0.01). In the qualitative analysis, the RPL group had a greater percentage of sperm aneuploidy, Conclusions:Qualitative analysis and quantitative analysis suggested an association between total sperm aneuploidy and RPL. However, for some specific type of sperm aneuploidy, male partners of women with a history of RPL had similar rates as fertile men. Further studies are needed owing to the significant heterogeneity between studies and lack of prospective pregnancy outcome data.
PUBLIC INTEREST STATEMENT
In order to determine whether there was a significant relationship between sperm aneuploidy and RPL-related abortions and to investigate whether sperm aneuploidy analysis may add useful information during reproductive counseling for couples with unexplained RPL,we performed a meta-analysis of male partners of women experiencing RPL. Our results showed that these men had significantly higher rates of sperm aneuploidy compared with male partners of fertile control women.For sperm aneuploidy to become a routine part of male RPL evaluation, it should be correlated with clinical outcomes; therefore, future studies are necessary to prospectively assess the impact of elevated sperm aneuploidy on miscarriage and live birth rates.
Competing Interests
The authors declares no competing interests.
1. Introduction
Recurrent pregnancy loss (RPL), which affects 1–2% of couples, is defined as having three or more consecutive pregnancy losses before week 20 of gestation (ESHRE Guideline Group on RPL, Citation2018). Owing to the complex causes of pregnancy loss and lack of evidence-based diagnostic strategies, the etiology of RPL remains unexplained in more than half cases (Gupta et al., Citation2007). Recent studies have focused on the effects of male factors, including routine semen analysis, sperm DNA fragmentation, Y chromosome microdeletions, and blood karyotype, on RPL (Bellver et al., Citation2010; Bhattacharya et al., Citation2008; Brahem et al., Citation2011; Carrell, Liu, et al., Citation2003; Gil-Villa et al., Citation2009, Citation2010; Puscheck & Jeyendran, Citation2007; Qiu et al., Citation2008). However, the contribution of men to recurrent miscarriage at the sperm chromosome level remains unclear. Thus, normal karyotyping of blood cells, sperm DNA fragmentation, and routine semen analysis cannot exclude the presence of chromosomal abnormalities in spermatozoa.
A direct evaluation of sperm chromosome complements could help improve our understanding of their roles in habitual abortion. Sperm chromosomal abnormalities include structural and numerical abnormalities, often observed with sperm aneuploidy. Sperm aneuploidy results from meiotic nondisjunction during oogenesis and sperm atogenesis, leading to abnormal gametes with disomies or nullisomies. However, whether the male gametes of couples with RPL exhibit higher rates of chromosomal anomalies than those of normal fertile men remains controversial.
Accordingly, in this study, we performed a systematic review and meta-analysis in order to determine whether there was a significant relationship between sperm aneuploidy and RPL-related abortions and to investigate whether sperm aneuploidy analysis may add useful information during reproductive counseling for couples with unexplained RPL.
2. Materials and methods
2.1. Search and selection strategy
An electronic literature search was performed using MEDLINE, Web of Science, Embase, and Cochrane library databases through 23 May 2019. The following terms were used in an automatic search: sperm aneuploidy, sperm aneuploidies, sperm aneuploid, sperm aneuploids, habitual abortion, habitual abortions, miscarriage, recurrent, recurrent miscarriage, recurrent miscarriages, abortion, recurrent, recurrent abortion, recurrent abortions, and recurrent early pregnancy loss. The detailed search strategy is shown in Table . Language was limited to English. Reference lists and relevant reviews were also manually searched to avoid omission of potential eligible studies. First authors and corresponding authors were contacted for additional information if necessary.
Table 1. Search strategy.(Web of science)
2.2. Inclusion and exclusion criteria
Two independent reviewers (Yifu Pu and Yujin Guo) identified and selected the articles according to the inclusion and exclusion criteria. The inclusion criteria were as follows: 1) observational studies; 2) studies focused on sperm aneuploidy and unexplained RPL (negative workup for repetitive miscarriage) between RPL groups with fertile control groups; 3) sufficient data were available; 4) normal parental karyotypes; 5) semen analyses included normal or abnormal; and 6) fluorescence in situ hybridization (FISH) was used for the analyses. The exclusion criteria were as follows: 1) animal studies; 2) meeting presentation or abstract; 3) languages other than English; 4) review, case report, clinical study (study design); 5) methods other than FISH; and 6) abnormal parental karyotypes.
2.3. Data extraction
Data were extracted from all eligible articles. The collected data included author/year, sample size, study type, definition of RPL, and sperm chromosome. Detailed eligible articles are shown in Table .
Table 2. Characters of included studiesTable2:Characters of included studies
2.4. Outcomes of interest
The following outcomes were obtained: total sperm aneuploidy in FISH, specific situations of aneuploidy (disomy 13/18/21/sex chromosomal/X/Y/XY), and diploidy.
2.5. Quality assessment
The included studies were critically assessed according to the Newcastle-Ottawa Quality Assessment Scale (NOS) (Wells et al., Citation2009), a valid tool recommended by the Cochrane Working Group to evaluate the risk of bias of nonrandomized studies. Studies were graded as low quality (0–3 points), medium quality (4–6 points), or high quality (7–9 points) according to the NOS score. Two reviewers (Yifu Pu and Xingwang Zhu) independently participated in the quality assessment. For disagreements, the authors discussed the studies to reach a consensus.
2.6. Statistical analysis
The odds ratios (ORs), standardized mean difference (SMD), and 95% confidence interval (CI) were used to estimate outcomes in RPL groups and fertile control groups. Pooled ORs and SMDs were calculated using the fixed-effects model, and heterogeneity between studies was measured by Q-tests and I2 statistics. Results with P values of less than 0.1 and I2 values of greater than 50% indicated significant heterogeneity, and a random-effects model was applied instead. Subgroup analyses were stratified according to chromosomal structure. Sensitivity analyses were performed to test the stability of the overall analysis by omitting single studies or changing the effects model. Publication bias was assessed using funnel plots. All statistical analyses were accomplished in Revman 5.3.
3. Results
3.1. Search results
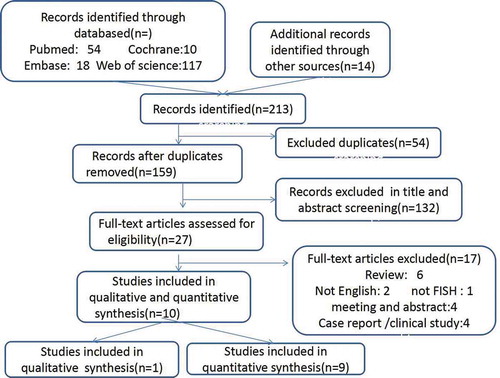
Search results are illustrated in the flow chart in Figure . In total, 199 articles were obtained from the electronic search, and 14 articles were obtained from reference lists or reviews. After title and abstract screening, 27 articles were identified for full text assessment. In further analyses,10 full-text studies were included in the current analysis(Table )
3.2. Study characteristics
One study was included for qualitative analysis owing to a lack of standard deviation data, and nine studies were included for quantitative analysis.326 male partners of women with recurrent pregnancy loss and 124 fertile men were included in this study.The characteristics of all 10 included studies are shown in Table . There studies reported the total sperm aneuploidy. Seven studies revealed sperm diploidy and disomy 18. Additionally, five studies showed disomy 21 and disomy X/Y/XY. Two studies reported disomy of sex chromosomes, and one study was analyzed qualitatively
3.3. Methodological quality
Assessment of risk of bias is illustrated in Table . Overall, four studies were graded as high quality. No studies were found to have low quality. Given the remarkable between-study heterogeneity of clinical characteristics, sensitivity analyses and publication bias assessment were conducted for each analysis.
Table 3. Assessment of risk of bias based on Newcastle-Ottawa Scale
3.4. Quantitative analysis
Statistical heterogeneity was found in the two subgroups. A random effects model was applied for pooling of the results. Meta results of the included studies is illustrated in Table .
Table 4. Meta results of the included studies
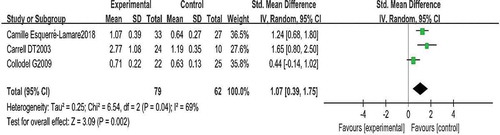
A comparison of total sperm aneuploidy is shown in Figures and . Three studies reported the total sperm aneuploidy for the two groups. Pooling the results of the three studies showed that the rate of total sperm aneuploidy was higher in patients with RPL than in controls (SMD: 1.07, 95% CI: 0.39–1.75, P < 0.01).
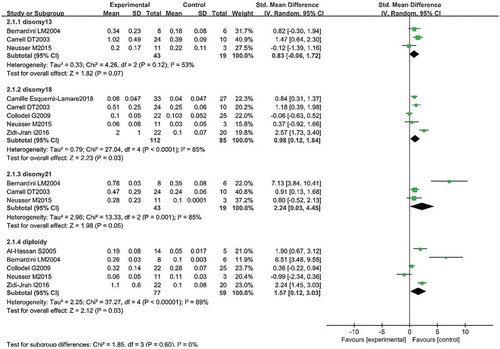
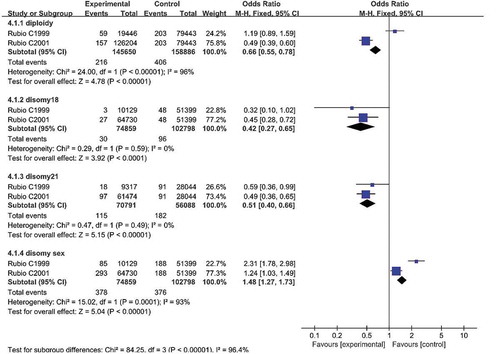
A comparison of sperm diploidy is shown in Figures , , and . Seven studies reported sperm diploidy in the two groups. Pooling the results of the seven studies showed that the percentage of sperm diploidy was higher in patients with RPL than in controls (SMD: 1.57, 95% CI: 0.12–3.03, P = 0.03; OR: 0.66, 95% CI: 0.55–0.78, P < 0.01).
A comparison of sperm disomy is shown in Figures , , and 6(c. Three studies reported the disomy 13 in two groups. Pooling the results of the three studies showed that the percentage of disomy 13 was similar in patients with RPL compared with controls (SMD 0.83; 95% CI: −0.06–1.72; P = 0.07).
Seven studies reported the disomy18 in two groups. Pooling the results of the seven studies showed that the percentage of disomy18 was higher in patients with RPL compared with controls (SMD: 0.98; 95% CI: 0.12–1.84; P = 0.03); OR 0.42 95% CI: 0.27–0.65; P < 0.01).
Five studies reported the disomy 21 in two groups. Pooling the results of the five studies showed that the percentage of disomy 21 was higher in patients with RPL compared with controls (SMD: 2.24, 95% CI: 0.03–4.45, P = 0.03; OR: 0.51, 95% CI: 0.40–0.66, P < 0.01).
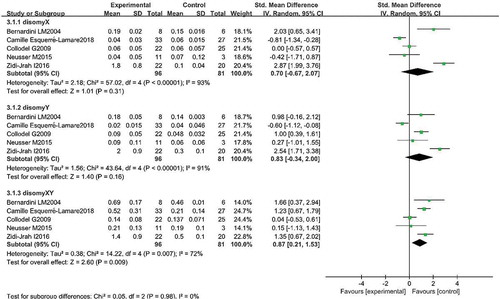
Comparison in others sperm disomy was shown in Figures , and 6(d. Five studies reported the disomy X in two groups. Pooling the results of the five studies showed that the percentage of disomy X was similar in patients with RPL compared with controls (SMD: 0.70, 95% CI: −0.67–2.07, P = 0.31).
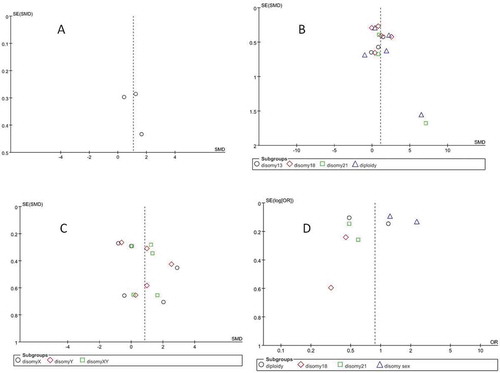
Figure 6. Funnel plots. (a) Sperm aneupoidy. (b) Sperm diploidy/disomy 13/18/21. (c) Sperm disomy X/Y/XY. (d) Sperm dipoidy/disomy 18/21/sex chromosome
Five studies reported the disomy Y in two groups. Pooling the results of the five studies showed that the percentage of disomy Y was similar in patients with RPL compared with controls (SMD: 0.83, 95% CI: −0.34–2.00, P = 0.16).
Five studies reported the disomy XY in two groups. Pooling the results of the five studies showed that the percentage of disomy XY was higher in patients with RPL compared with controls (SMD: 0.87, 95% CI: 0.21–1.53, P < 0.01).
Two studies reported the disomy sex chromosome in two groups. Pooling the results of the two studies showed that the percentage of disomy sex chromosome was higher in patients with RPL compared with controls (SMD: 1.48, 95% CI: 1.27–1.73, P < 0.01).
3.5. Qualitative analysis
One study was included for qualitative analysis owing to a lack of standard deviation data. The results showed that men with RPL had a greater percentage of sperm aneuploidy within the sex chromosomes and within chromosomes 18 and 13/21 (1.04% versus 0.38%; 0.18% versus 0.03%; 0.26% versus 0.08%, respectively). Thus, men with RPL had increased sperm aneuploidy compared with controls. The study was generally consistent with the results of quantitative analysis.
3.6. Synthesis of results
One study did not report standard deviations of outcomes in the original articles. Nine studies were included in the quantitative analysis. In total, 450 men were included in this review (326 [72.4%] partners of women with RPL and 124 [27.6%] fertile men). From a pooled analysis of the primary outcome, we found that male partners of women with RPL had a significantly higher rate of sperm aneuploidy than those of fertile control women (SMD: 1.07, 95% CI: 0.39–1.75). Statistical heterogeneity was considerable. Funnel plot and subgroup analyses were performed according to different chromosomes. The statistical heterogeneity remained high, despite subgroup analysis. Some variables, including the criteria applied for RPL definition, such as minimum number of consecutive abortions (two versus three), or criteria adopted for scoring signals during FISH (including or not including the nullisomy rates into the calculations), and the definition of semen quality, may have affected the results. Sample size and technical reasons may also affect the results. For example, sperm sample processing, the FISH probe used, or different inclusion criteria (e.g., men with normal or abnormal semen parameters) could lead to variations. Notably, for some specific types of sperm aneuploidy (disomy 13/Y/X), male partners of women with a history of RPL had similar rates of aberrations compared with fertile men. For other specific situations, such as disomy 18/21/XY/sex chromosomes, although the conclusions were relatively consistent, more research is needed to obtain results with more reliability. Therefore, the conclusions reached herein should be interpreted with caution. Notably, total sperm aneuploidy included disomy 1–22/sex chromosomes and nullisomy 1–22/sex chromosomes. Consequently, for specific types of chromosome aberrations, the conclusions may not be consistent with the total sperm aneuploidy. Thus, further studies are needed to demonstrate the accuracy of the results by testing all types of chromosome aneuploidies.
4. Discussion
In this review, we performed a meta-analysis of male partners of women experiencing RPL. Our results showed that these men had significantly higher rates of sperm aneuploidy compared with male partners of fertile control women. Although there was consistently elevated sperm aneuploidy in the RPL population, there was considerable heterogeneity among studies. Several factors likely contributed to the observed heterogeneity. First, there are multiple definitions of idiopathic RPL. In addition, multiple chromosomes can be evaluated, including chromosomes 13, 18, and 21 and sex chromosomes. However, analysis of total sperm aneuploidy should include all types of chromosome aneuploidy. Third, some studies used normal semen samples, and others used abnormal semen samples. Each of these factors may have significantly affected total sperm aneuploidy in the individual studies as well as the heterogeneity of the meta-analysis.
The most important limitation of this review is that the 10 studies were carried out in different countries using different research strategies. Additionally, the studies did not include all types of chromosome aneuploidy. These limitations may have caused obvious heterogeneity. Another limitation was the small number of included trials and the small number of participants in each of these trials. Because of these limitations, publication bias was assessed using funnel plot analysis with a qualitative approach.
One strength of the study was that we did not exclude studies based on research approach. Additionally, the included trials showed high quality and precision. Other studies (Caseiro et al., Citation2015; Giorlandino et al., Citation1998; Lou et al., Citation2013; Rosenbusch et al., Citation1991) were mostly consistent with our current findings. These studies showed that the incidence of aneuploidy was increased in individuals with a history of repeated abortion. Their findings provided insights into the potential causes of RPL and showed that the reported cases may help improve reproductive outcomes. Moreover, from our analysis, we found that this current report is the first systematic review and meta-analysis of sperm aneuploidy and RPL.
Notably, the incidence of aneuploidy in the RPL group was increased compared with that in the control group, but was still relatively low. Generally, only women are subjected to testing for RPL, whereas men have not been evaluated sufficiently. In particular, marked sperm aneuploidies could represent a significant cause of infertility. We suggest that all chromosomes should be studied to obtain a comprehensive view of aneuploidy frequencies in spermatozoa from couples with RPL. If a higher incidence of specific chromosome abnormalities is found, decision making regarding the choice of treatment strategies, such as insemination with donor sperm or pre-implantation genetic diagnosis would be improved. For sperm aneuploidy to become a routine part of male RPL evaluation, it should be correlated with clinical outcomes; therefore, future studies are necessary to prospectively assess the impact of elevated sperm aneuploidy on miscarriage and live birth rates.
Author contributions
Yifu Pu designed the study; Yifu Pu and Yujin Guo performed the literature search; Yifu Pu and Xingwang Zhu analyzed the data; Yifu Pu wrote the paper; Lei Yan and Shaoming Lu provided critical feedback and revised the manuscript. All authors approved the final draft of the manuscript.
Declaration of competing interest
No potential conflict of interest was reported by the authors.
Acknowledgements
We thank all the individuals who assisted with literature collection, manuscript compilation, and other tasks.
Additional information
Funding
Notes on contributors
Shaoming Lu
The author’s name is Pu Yifu, a graduate student in obstetrics and gynecology (Human Reproduction) at Shandong University. My research areas include recurrent miscarriage, sperm DNA fragments, sperm aneuploidy, eclampsia, and unicornuate uterus. I am good at writing meta analysis and clinical prediction nomogram and other related papers. So far, 2 sci papers and 2 Chinese papers have been accepted.
My E-mail: [email protected]/[email protected]
Telephone:15953143277
Address:jingqiweibaRD,jinan250000,shandong province,China
References
- Al-Hassan, S., Hellani, A., Al-Shahrani, A., Al-Deery, M., Jaroudi, K., & Coskun, S. (2005). Sperm chromosomal abnormalities in patients with unexplained recurrent abortion. Archives of Andrology, 51(1), 69–13. https://doi.org/10.1080/014850190518062
- Bellver, J., Meseguer, M., Muriel, L., Garcia-Herrero, S., Barreto, M. A., Garda, A. L., Remohí, J., Pellicer, A., & Garrido, N. (2010). Y chromosome microdeletions, sperm DNA fragmentation and sperm oxidative stress as causes of recurrent spontaneous abortion of unknown etiology. Human Reproduction, 25(7), 1713o1721. https://doi.org/10.1093/humrep/deq098
- Bernardini, L. M., Costa, M., Bottazzi, C., Gianaroli, L., Magli, M. C., Venturini, P. L., Francioso, R., Conte, N., & Ragni, N. (2004). Sperm aneuploidy and recurrent pregnancy loss. Reproductive Biomedicine Online, 9(3), 312–320. https://doi.org/10.1016/s1472-6483(10)62147-5
- Bhattacharya, S. M. (2008). Association of various sperm parameters with unexplained repeated early pregnancy loss–which is most important? International Urology and Nephrology, 40(2), 391–395. https://doi.org/10.1007/s11255-007-9282-y
- Brahem, S., Mehdi, M., Landolsi, H., Mougou, S., Elghezal, H., & Saad, A. (2011). Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology, 78(4), 792–796. https://doi.org/10.1016/j.urology.2011.05.049
- Carrell, D. T., Liu, L., Peterson, C. M., Jones, K. P., Hatasaka, H. H., Erickson, L., & Campbell, B. (2003). Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Archives of Andrology, 49(1), 49–55. https://doi.org/10.1080/01485010290099390
- Carrell, D. T., Wilcox, A. L., Lowy, L., Peterson, C. M., Jones, K. P., Erickson, L., Campbell, B., Branch, D. W., & Hatasaka, H. H. (2003). Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstetrics and Gynecology, 101(6), 1229–1235. https://doi.org/10.1016/s0029-7844(03)00339-9
- Caseiro, A. L., Regalo, A., Pereira, E., Esteves, T., Fernandes, F., & Carvalho, J. (2015). Implication of sperm chromosomal abnormalities in recurrent abortion and multiple implantation failure. Reproductive Biomedicine Online, 31(4), 481–485. https://doi.org/10.1016/j.rbmo.2015.07.001
- Collodel, G., Giannerini, V., Antonio Pascarelli, N., Federico, M. G., Comodo, F., & Moretti, E. (2009). TEM and FISH studies in sperm from men of couples with recurrent pregnancy loss. Andrologia, 41(6), 352–360. https://doi.org/10.1111/j.1439-0272.2009.00936.x
- ESHRE Guideline Group on RPL. (2018). ESHRE guideline: Recurrent pregnancy loss. Human Reproduction Open, 2018(2), hoy004. https://doi.org/10.1093/hropen/hoy004
- Esquerré-Lamare, C., Walschaerts, M., Chansel-Debordeaux, L., Moreau, J., Bretelle, F., Isus, F., Karsenty, G., Monteil, L., Perrin, J., Papaxanthos-Roche, A., & Bujan, L. (2018). Sperm aneuploidy and DNA fragmentation in unexplained recurrent pregnancy loss: A multicenter case-control study. Basic and Clinical Andrology, 28(1), 4. https://doi.org/10.1186/s12610-018-0070-6
- Gil-Villa, A. M., Cardona-Maya, W., Agarwal, A., Sharma, R., & Cadavid, A. (2009). Role of male factor in early recurrent embryo loss: Do antioxidants have any effect? Fertility and Sterility, 92(2), 565e571. https://doi.org/10.1016/j.fertnstert.2008.07.1715
- Gil-Villa, A. M., Cardona-Maya, W., Agarwal, A., Sharma, R., & Cadavid, A. (2010). Assessment of sperm factors possibly involved in early recurrent pregnancy loss. Fertility and Sterility, 94(4), 1465r1472. https://doi.org/10.1016/j.fertnstert.2009.05.042
- Giorlandino, C., Calugi, G., Iaconianni, L., Santoro, M. L., & Lippa, A. (1998). Spermatozoa with chromosomal abnormalities may result in a higher rate of recurrent abortion. Fertility and Sterility, 70(3), 576–577. https://doi.org/10.1016/s0015-0282(98)00192-7
- Gupta, S., Agarwal, A., Banerjee, J., & Alvarez, J. G. (2007). The role of oxidative stress in spontaneous abortion and recurrent pregnancy loss: A systematic review. Obstetrical and Gynecological Survey, 62(5), 33547. https://doi.org/10.1097/01.ogx.0000261644.89300.df
- Lou, C., Qiang, R., Yan, H. M., Li, N., & Li, J. (2013). Study on the relationship between sperm cell chromosome aneuploid detected by FISH and recurrent abortion. Zhong Guonankexuezazhi, 2, 33–37. https://doi.org/http://doi:10.3969/j..1008-0848.2013.02.008
- Neusser, M., Rogenhofer, N., Durl, S., Ochsenkuhn, R., Trottmann, M., Jurinovic, V., Steinlein, O., von Schönfeldt, V., Müller, S., & Thaler, C. J. (2015). Increased chromosome 16 disomy rates in human spermatozoa and recurrent spontaneous abortions. Fertility and Sterility, 104(5), 1130–1137. https://doi.org/10.1016/j.fertnstert.2015.07.1160
- Puscheck, E. E., & Jeyendran, R. S. (2007). The impact of male factor on recurrent pregnancy loss. Current Opinion in Obstetrics and Gynecology, 19(3), 222n228. https://doi.org/10.1097/GCO.0b013e32813e3ff0
- Qiu, Y., Wang, L., Zhang, L., Yang, D., Zhang, A., & Yu, J. (2008). Analysis of chromosomal abnormalities and sperm DNA fragmentation in infertile males. China Journal of Medical Genetics, 25, 681–685. PMID: 19065532
- Ramasamy, R., Scovell, J. M., Kovac, J. R., Cook, P. J., Lamb, D. J., & Lipshultz, L. I. (2015). Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertility and Sterility, 103(4), 906–909. https://doi.org/10.1016/j.fertnstert.2015.01.029
- Rosenbusch, B., Sterzik, K., & Lauritzen, C. (1991). Cytogenetic analysis of sperm chromosomes in couples with habitual abortion. Geburtshilfe und Frauenheilkunde, 51(5), 369–372. https://doi.org/10.1055/s-2007-1026160
- Rubio, C., Gil-Salom, M., Simon, C., Vidal, F., Rodrigo, L., Minguez, Y., Remohí, J., & Pellicer, A. (2001). Incidence of sperm chromosomal abnormalities in a risk population: Relationship with sperm quality and ICSI outcome. Human Reproduction, 16(10), 2084–2092. https://doi.org/10.1093/humrep/16.10.2084
- Rubio, C., Simon, C., Blanco, J., Vidal, F., Mínguez, Y., Egozcue, J., Crespo, J., Remohí, J., & Pellicer, A. (1999). Implications of sperm chromosome abnormalities in recurrent miscarriage. Journal of Assisted Reproduction and Genetics, 16(5), 253–258. https://doi.org/10.1023/a:1020315529090
- Wells, G., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2009). The Newcastle-Ottawa Scale (NOS) for assessing the quality if non randomized studies in meta-analyses. Epub Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- Zidi-Jrah, I., Hajlaoui, A., Mougou-Zerelli, S., Kammoun, M., Meniaoui, I., Sallem, A., Brahem, S., Fekih, M., Bibi, M., Saad, A., & Ibala-Romdhane, S. (2016). Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertility and Sterility, 105(1), 58–64. https://doi.org/10.1016/j.fertnstert.2015.09.041