Abstract
Cancers remain one of the leading causes of death in the world. However, known conventional cancer medicines remain scarce, expensive and out of reach to many patients. To provide an alternative for the expensive therapies, plants are proving to have potential to lead to the development of more drugs against cancers. In this work, we aimed at isolating possible pure compounds from Eichhornia crassipes roots and leaves, examining their prospects to be developed into oral drugs by looking at their chemical properties and also exploring their cytotoxic activities. A total of 12 isolates (A-L) were obtained and examination of their UV and mass profiles on LCMS and cytotoxicity on human cell lines. Compound C was the most active against MCF-7 cell lines (3.08 ± 0.06 µg/ml) and (3.92 ± 0.06 µg/ml) against HepG2. The standard drug, doxorubicin, had an IC50 value of 0.29 ± 0.05 µg/ml against MCF-7 and 0.33 ± 0.04 µg/ml against HepG2 cell lines. UV results from the LCMS chromatograms showed that some active isolates do not possess the chemistry suitable to be developed into oral drugs as they are while others that are not as potent possess very good drug-like chemistry. We therefore conclude that there is need for drug developers to consider chemical properties and medicinal chemistry prospects of active isolates/compounds through rational optimization in plant-based drug discovery. Eichhornia crassipes should be considered as a cheap source of potential drug hits against cancers.
Public Interest Statement
This work focussed on the potential therapeutic properties of isolates from water hyacinth that was collected from the Shire River in Malawi. This plant is common on many calm water bodies including on Lake Victoria in central Africa. Not much has been done one the plant and the team thought of studying and contributing knowledge about what some chemical constituents of the plant’s roots and leaves separately can do on some breast and hepatocellular cancer cells. With the understanding that most natural product research on the African continent is done upto the level of testing extracts on various biological targets, the work sought to go beyond the extract level by isolating compounds and going a little further to characterize them using chromatographic and spectroscopic techniques which provided ideas about the cytotoxic compound’s masses and UV absorptive chromophores in the roots and leaves of the plant. Medicinal chemistry aspects of the isolates were discussed to guide developers on future projects.
1. Introduction
Incidences of cancers are rising world-over, getting closer to becoming the latest developing countries’ epidemic (Makhafola & McGaw, Citation2017). In the past, high mortality from cancers was reported in North America, New Zealand and the rest of the world (Parkin, Citation2004) but currently, the burden in Africa is getting large (Azubuike et al., Citation2018) partly due to poor diagnostic systems, weaker economic potential to afford drugs and general lack of preparedness (Makhafola & McGaw, Citation2017). The most common cancers in Africa include cancers of the breast, liver, cervix, colon, rectum, oesophagus, stomach, lungs and bladder (Makhafola & McGaw, Citation2017). Known conventional cancer medicines remain scarce, expensive and out of reach to many patients particularly in the developing world. Cases of cancer and their associated deaths have been projected to have an almost two-fold rise by the year 2030 according to the International Agency for Research on Cancer (IARC)(Stewart et al., Citation2015) and it is terrifying to note that its burden does not only rest on the patients but also extends to caregivers (Ge & Mordiffi, Citation2017; Johansen et al., Citation2017) adversely affecting socio-economic development. Despite this, there is a decrease in resourcing the fight against cancers in many African countries as much emphasis is put on communicable diseases and ailments such as HIV, TB, malaria and other infectious diseases (Makhafola & McGaw, Citation2017). Besides commonly known lifestyle as a contributing factor to cancer incidences (Torre et al., Citation2015), A good number of cancer cases (~16%) are infection-caused (Cancer_Genome_Atlas_Network, Citation2012) and the infections that lead to cancers are reported to be more prevalent in developing countries (Makhafola & McGaw, Citation2017). In this regard, there is need to keep on the fight against cancers through rational drug discovery and development from more available and cheaper resources such as plants.
1.1. Eichhornia crassipes
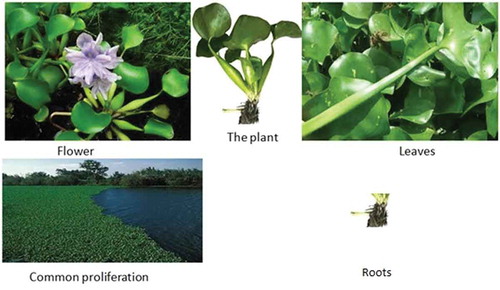
Plants have always played a significant role in the search for newer drugs including anticancer drugs. In 2013, at least half of drugs on the market, including anticancer drugs, were natural products derived (Pan et al., Citation2013). Eichhornia crassipes (pontederiaceae) is one of such plants that provide potential sources of compounds that can be used as they are or optimized against cancers. Eichhornia crassipes is a very common waterweed known as water hyacinth (Ekiddo in Luganda of Uganda and Namasipuni in Chichewa of Malawi) found naturally floating on waterbody surfaces and sometimes directly rooted into the ground in waterbodies. The plant is locally used against cancers and chronic wounds in some parts of Malawi and Uganda and is known to be scientifically potent against these (S. Kumar et al., Citation2014). shows a cross-section of the plant.
The plant is reported to have various health benefits including nutrients, mineral metals, antimicrobial activities, antioxidant activities as well as cytotoxic activities (Haggag et al., Citation2017; S. Kumar et al., Citation2014). Previous research has shown that hexane: ethylacetate extracts and some isolated compounds from E. crassipes’ leaves were active against cancers (Mtewa et al., Citation2018),(Aboul-Enein et al., Citation2014), (Tyagi & Agarwal, Citation2017). Research has also demonstrated that chemical content of the roots may not always be the same as that of the leaves for plant species (Haggag et al., Citation2017) due to differences in the rates and extents of metabolic processes as well as influence of the immediate surrounding environments. This necessitates conducting similar research on different parts of plants to explore the phytochemistry of the plants as widely as possible.
1.2. Chromatographic analysis
Chromatographic techniques help in the separation and purification of compounds depending on the advancement of the level of the tools used. One aspect that is of a great interest in the current work is that chromatographic techniques are able to give chromatogram of compounds that are capable of showing both the purity and the relative polarity of the compounds (McMaster, Citation2007). It is therefore possible to predict the possibilities of molecules to be developed further for various purposes such as drug development partly based on their polarity. Drugs that are too polar tend to fail to pass through biological membranes and if they do, they also have too high clearance and in the same way, compounds that are too non-polar tend to get stuck within biological membranes and they fail to effectively cross membranes, both ways, resulting into poor bioavailability (Bowe et al., Citation1997; Kern & Di, Citation2008; Mtewa, Sesaazi, Lampiao et al., Citation2020).
1.3. Cytotoxicity assays
Cytotoxicity assays on cancer cell lines are conducted using various means. The most basic and commonly used assays include the 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT), Trypsin blue and sulforhodamine B (SRB) assays. According to original SRB assay development paper (Philip Skehan et al., Citation1990), the SRB assay focuses on the concentration of protein in the assay, meaning that the more cells get degraded or denatured, the higher reduction of the dye absorbance since their protein gets turned into amino acids. MTT assay is a water-soluble assay and is generally dependent on the activities of cellular dehydrogenase and on the other side. With this in mind, MTT can be more useful to determine antiproliferative effects while SRB can be more useful for inhibitory effect determinations. The common feature in the assays is the reduction of test dyes upon the incubation of cultured cell lines with test compounds or extracts which is determined calorimetrically using a spectrophotometer. It is reported from some research findings that MTT assay should be undertaken with caution as it somehow increases and sometimes decreases the activity of extracts (Jo et al., Citation2015; Karakaş et al., Citation2017). The increasing and decreasing activity findings were obtained from alcohol extracts (MeOH and EtOH, respectively) and suggest that this could be due to the interference between MTT and some other ingredients in the extracts. The observation may not apply to pure samples completely dried and void of methanol and/or ethanol which are some of the cautionary measures to be taken when undertaking this assay. Such a limitation does not render it unreliable as it is still considered one of the most popular, versatile (P. Kumar et al., Citation2018) and sensitive assays (Aboul-Enein et al., Citation2014) that give a direction on the cytotoxicity of samples on cell lines to date. Both MTT and SRB assays are still relevant for use provided appropriate cautionary measures need to be taken. However, it is still recommendable to undertake confirmatory experiments using other assays for extracts where possible which would be very critical particularly for late-stage optimized drug leads.
According to a systematic review on the anticancer properties of the plant in 2018, it was noted that anticancer property research on the plant is very limited (A. G. Mtewa et al., Citation2018). There is therefore a need to explore more compounds using more solvent systems as well as targeting other parts of the plant in addition to leaves that have been studied before. The aim of this study was to isolate pure compounds from a wider solvent spectrum from Hexane through Ethyl acetate, dichloromethane to methanol extracts of both roots and leaves of E. crassipes, determine their molecular masses and cytotoxicity using liver cancer cell lines (HepG2) and breast cancer cell lines (MFC7). Findings from this work will be a useful guide to drug discoverers on the nature of cytotoxic compounds to be expected from both roots and leaves of the plant in terms of polarity, dose–response and size of some isolated molecules which are some of the important properties in drug discovery at a lead optimization stage.
2. Materials and methods
2.1. Reagents and materials
Methanol, n-Hexane, Ethylacetate and Dichloromethane were purchased from Sigma-Aldrich (Germany). Doxorubicin was purchased from Sigma (Missouri, USA). Thin-layer chromatography (TLC-F254) and Trypsin-EDTA were obtained from Merck (Darmstadt, Germany). MCF-7, HepG2 cell lines and fetal bovine serum were obtained from ATCC (England, UK), PerkinElmer Victor X3 Multimode plate reader was manufactured by PerkinElmer Inc. (Massachusetts, USA) and LCMS manufactured by Agilent technologies (California, USA).
2.2. Plant collection and identification
Eichhornia crassipes whole plants were collected from Shire river in Liwonde, Malawi within the following location: 15°03ʹ24.9”S 35°13ʹ13.4”E, 15°03ʹ12.9”S 35°13ʹ13.2”E, 15°03ʹ07.5”S 35°13ʹ15.4”E and 15°03ʹ11.3”S 35°31ʹ12.5”E between February and March, 2019. The plant species was identified by a Malawi Herbarium and Botanic gardens (MHBG) botanist, Mr. Hassam Patel. The plant is available at the MHBG under accession numbers 35963 and 35964.
2.3. Plant preparation
The method was conducted as described in the literature (Aboul-Enein et al., Citation2014; Xie et al., Citation2011) with minor modifications to suit the materials available. Separately, root and leaf plant material was continuously washed using tap followed by distilled water. The samples were air-dried in a well-ventilated room at room temperature for 4 weeks, ground to powder then stored at −10°C until use. The extraction processes were carried out several times to acquire meaningful amount for use but at each run, about 500 g of the plant material was extracted three times in 2.5-l methanol over a 24 h period in a dark cupboard. The extracts were concentrated on a rotor evaporator at 40°C.
2.4. Isolation, purification and mass determination
The dried methanol extract (2 g) was then run on a manual column in the following gradient systems: Hexane: Ethylacetate (100:0, 95:05, 90:10, 85:15, 80:20, 75:25, 70:30, 60:40, 50:50) and Dichloromethane: Methanol (100:0, 95:05, 90:10, 85:15). The sub-fractions obtained were continuously checked on silica gel pre-coated thin layer chromatography (TLC-F254) under normal light as well as UV light (254/365 nm) for purity. Those that appeared to have a similar retention time were combined and those that were still not pure (showing more than one spot) were purified further using a much smaller 10 ml volume syringe column where possible using appropriate gradient systems as guided by the TLC profiles until a single spot on TLC was obtained for each sub-fraction. Purity was confirmed using a liquid chromatography-mass chromatography (LCMS, Agilent) UV detector. Mass of pure isolates was obtained using LCMS (Agilent) running at a gradient of 5–95% acetonitrile/water +1% formic acid and prepared as described by (Baragaña et al., Citation2016). In this study, LCMS was used to determine only purity and UV detection ranges and molecular masses of the isolates from 100 to 1400 m/z range.
2.5. Anticancer activities
Only pure compounds that were in reasonably enough amounts to be carried forward for the next experiments were used in the determination of anticancer activities of the isolates.
2.6. Cell culture
This method was carried out as done in literature (Freshney, Citation2002). From liquid nitrogen storage, breast cancer cell lines (MCF7) and Human hepatocellular cancer cell lines (HepG2) were developed and maintained in RPMI 1640 culturing medium together with penicillin (100units/ml), streptomycin (100 µg/ml) and fetal bovine serum (10%) in a 37°C, 5% CO2 environment.
2.7. Cytotoxicity assay
This method was undertaken as described in the literature (Skehan et al., Citation1990). Trypsin-EDTA (0.25%) was used to collect MCF7 and HepG2 cells which were then plated in a 96-well plate at 1.0 × 103–2.0 x 103 cells per well. Separately, individual plant isolates and standard drug (doxorubicin) were added to the wells and incubated for 72 hrs. TCA (10%) was added to the wells at 4°C and rested for 1 h. The cells were washed then added to 4% of the dye in a dark cupboard then after 10 minutes, washed using glacial acetic acid (1%). The cells were left to dry overnight followed by the addition of Tris-HCl to dissolve the cells that were by now stained with the SRB dye. The intensity of the dye in the cells was then measured at 540 nm using a PerkinElmer Victor X3 Multimode plate reader. Dose–response curves and IC50 values were generated using Graphpad Prism 5.
3. Results
3.1. Pure isolates from E. crassipes extracts
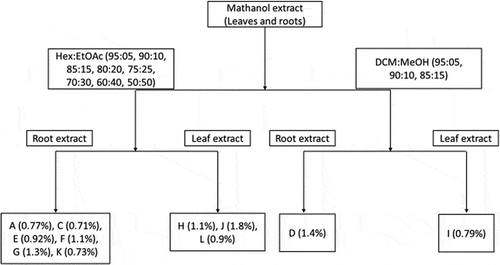
A minimum of 65 test tubes were obtained in each solvent gradient isolation experiment (DCM:MeOH and Hex:EtOAc for each of the roots and leaves’ extracts).
shows some of the subfractions that were purified further at a smaller scale using syringes.
After further purifications at smaller scale (syringes or burettes), 12 pure compounds (A-L) were isolated from extracts as shown in . Due to their small amounts, compounds K and L were tested only against MCF-7 and not HepG2. Despite having more compounds isolated from roots in this study, the authors would like to make it clear that it does not imply that the roots have more compounds, but these were some of those they could comfortably isolate in their best pure forms with reasonably enough yield to proceed to further experiments.
3.2. Determination of purity and polarity of the isolates
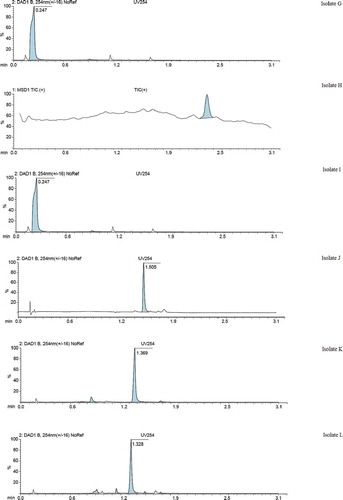
Liquid Chromatography-Mass Spectroscopy’s UV detector (Agilent) was used to ascertain the purity of the isolates. Figure 4 partly shows UV chromatograms of the isolates.
3.3. Determination of molecular mass of isolates
LCMS (agilent) was used to determine the molar masses of the isolates. The other part of Figure 4 shows the mass spectra of the isolates.
Cytotoxicity assays
Figure 4: Cytotoxicity of pure isolates on MCF-7 and HepG2 cell lines. The red-coloured results are for MCF-7 and those in black are for HepG2 cell lines.
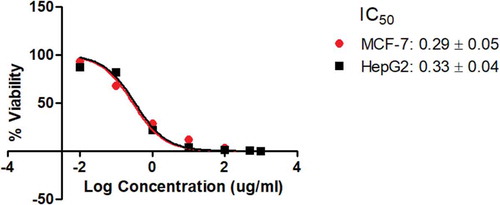
: Dose response curve for the standard drug, doxorubicin.
Supplementary material 1 shows the normalization of data transform for the standard drug doxorubicin in Graphpad prism.
4. Discussion
The difference between this work and previous anticancer isolation works on the same plant (Aboul-Enein et al., Citation2014) is that the current work has to include isolation from roots, linked LCMS profiles with physicochemical and pharmacokinetic properties of the compounds isolated based on polarity and has also managed to widen the solvent gradient spectrum to allow for more possible isolations than in previous works. Although this work did not proceed to elucidate the structures of these isolates due to limited yields realised from the processes, it is a step forward from just examining extracts. This work reported the cytotoxicity of Eichhornia crassipes isolates against HepG2 and MCF-7 cell lines separately from both roots and leaves over the wider spectrum of polarities and determined their molecular masses and examined their UV profiles in a medicinal chemistry perspective for the first time. The activities of some of the isolates were all higher than that of doxorubicin which showed very high activity against both cell lines, which was expected as previous works reported it to be a very active anticancer drug (Hu et al., Citation2009; Thorn et al., Citation2011). The generated dose–response curves can hardly show error bars because the errors were too small to appear on the curves. Supplementary material 1 shows the normalization of Transform of Data for the standard drug doxorubicin in Graphpad prism which includes the errors generated.
The results from this study reinforce earlier findings that Eichhornia crassipes could be a plant to look at in the future of fighting diseases such as cancers (Mtewa et al., 2018). According to literature (Aboul-Enein et al., Citation2014), isolates with IC50 values of lower than 1 µg/ml are considered strongly active as doxorubicin was found to be in , above 1 µg/ml but less than 10 µg/ml as moderately potent and above 10 µg/ml as weakly active. In this work, a moderately active compound C was the most active against MCF-7 cell lines (3.08 ± 0.06 µg/ml) which also had a moderate activity against HepG2 (3.92 ± 0.06 µg/ml) compared to the standard drug in (0.29 ± 0.05 µg/ml against MCF-7 and 0.33 ± 0.04 µg/ml against HepG2 cell lines). However, the most active isolate against HepG2 was isolate G (3.39 ± 0.05 µg/ml). The results show that the activity of the same isolate on both HepG2 and MCF-7 was not very different; however, this cannot be used to draw conclusions to generalize this observation as other isolates within this study show the possibility of having very different activity levels of one isolate against the two cell lines such as compound B, F, H and J. The close values observed in this study could only be a coincidence as different enzyme acceptors on different cell lines are expected to have different cavities on their binding sites (Flynn, Citation2007; Mtewa et al., Citation2018; Patrick, Citation2017).
Considering that the study of compound polarity is crucial in predicting possibilities in developing it further into oral drugs in drug discovery and development. Besides polarity, there are key factors that should always be kept in mind if drugs are to be realised from plants. These include bioactivity, molecular size, lipophilicity and solubility among others (Mtewa et al., Citation2018; Pan et al., Citation2013). According to Lipinski's rule of 5, a molecule can easily be made into an oral drug if it shows some bioactivity, has a mass of less than 500 g/mol (Di et al., Citation2009; Rostron, Citation2020) and has a reduced and well-balanced lipophilicity and solubility, properties that have a high correlation to polarity (Schneider, Citation2013) that has been evaluated in the current work. Looking further at the chromatograms and mass spectra of isolates C and G (Figure 4), they would both be good candidates if only mass (<500 g/mol) was to be considered. However, isolate G is observed to be too polar to be considered for oral drug candidacy. It would require some more work to have it restructured most likely by adding some bits of fatty substituents to it to improve its permeability controls and ligand efficiency (LE) prospects (Di et al., Citation2009; Schneider, Citation2013).
It is interesting to note that some compounds could have a very low bioactivity but their structural properties are very good for consideration for oral drug development. In this work, such is the case of isolate B which is very poorly active (19.71 ± 0.09 µg/ml) against MCF-7 but appears to have a good balance in terms of polarity as evident from the elution time on its chromatogram which would be a good feature for a promising lipophilic value as well as other associated drug-like properties. Such a molecule would have a good partition coefficient between fatty and aqueous phases of membranes and carries a good chance to survive pharmacokinetics and pharmacodynamics in the body (Di et al., Citation2009; Patrick, Citation2017; Schneider, Citation2013). This is a good case that shows that not all active extracts, fractions or isolates would automatically make a good oral drug candidate, others may need to be optimized further to have their physicochemical and pharmacokinetic properties modified towards hit or lead characteristics. Poor or lack of proper optimization of drug-like properties have led to drug development project failures with huge financial and time costs in many cases and can be avoided by looking beyond bioactivity of extracts, fractions and/or pure isolates (Hutchinson & Kirk, Citation2011; Moreno & Pearson, Citation2013; Takebe et al., Citation2018).
5. Conclusion
This study does not only examine the relevance and promise that Eichhornia crassipes provides in therapeutic studies but also shows that both the upper (leaves) and the lower (roots) parts of the plant possess such potential uses. One of the most intriguing aspects is that good bioactivity does not necessarily guarantee a good oral drug molecule as some very active molecules could be in either extremes of polarity which cannot make good oral drugs but with some more work on structural modification, polarity, ionizability and other properties, which have a strong bearing on compound lipophilicity, one of the most important, if not the central parameter in drug optimization, can be developed into a good oral drug molecule without need for or with minimal need for careers and other artificial transporters. The authors recommend that researchers, especially in phytomedicine should strive to go an extra mile for every active extract, fraction, sub-fraction or compound to determine if the “drug” in question could be worth spending resources on at the earliest stages as possible as they consider a clinical trial of an oral candidate in future. Standardization and optimization of herbal isolates could be a good starting point. An interdisciplinary approach to drug discovery is therefore recommended to effectively and efficiently see success in drug discovery from plants through the pipelines to the end. Failure to check and optimize properties at this early stage ends up in attrition which has huge costs to research projects.
Author Contributions
AGM conceptualized the work, did the analyses and drafted the manuscript. KJN, FL, ELP and DCS directed the work in the laboratory. AW, CUT and PEO and the rest of the authors reviewed the manuscript.
Conflicts of Interest
The authors declare no conflicts of interests.
Supplemental Material
Download MS Word (14.1 KB)Acknowledgements
Authors acknowledge ACEPHEM at the University of Malawi’s College of Medicine for providing facilities for early stages of the work, Egerton University in Kenya and the DAAD from Germany for providing spectroscopic facilities. The authors acknowledge Mr. Reuben Maghembe of the University of Dar-Es-Salaam for assisting in the microbiology part and Mr. Happy Nyirongo and Joseph Kumphanda for assisting in the chemistry labs. The authors also acknowledge Dr. Lauren Webster of the University of Dundee and the WCAIR for intensive training in medicinal chemistry for AM.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Notes on contributors
Andrew G. Mtewa
The authors are involved in a wide range of research projects with a multidisciplinary approach in drug discovery and development. The team comprises a Medicinal Chemist, Synthetic Organic Chemist, Conservationist, Physiologist, Pharmaceutical Chemist, Pharmacists and a Pharmacologists spread over more than 30 years of research experience, scholarship and publications. This puts together various skills and knowledge to make sense of contemporary techniques in drug discovery including pharmacognostic aspects. The wider scope of the focus of the team surrounds the search for potential drug hits, leads and extracts that promise significant traits worth pursuing down the discovery pipeline. It combines in vitro, in vivo and in silico experiments and strive to go beyond ‘just extracts’ in its scientific studies. This paper is part of the work that the team did on Eichhornia crassipes.
References
- Aboul-Enein, A. M., Shanab, S. M., Shalaby, E. A., Zahran, M. M., Lightfoot, D. A., & El-Shemy, H. A. (2014). Cytotoxic and antioxidant properties of active principals isolated from water hyacinth against four cancer cells lines. BMC Complementary and Alternative Medicine, 14(397), 11. https://doi.org/10.1186/1472-6882-14-397
- Azubuike, S. O., Muirhead, C., Hayes, L., & McNally, R. (2018). Rising global burden of breast cancer: The case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: A review. World Journal of Surgical Oncology, 16(1), 63. https://doi.org/10.1186/s12957-018-1345-2
- Baragaña, B., Norcross, N. R., Wilson, C., Porzelle, A., Hallyburton, I., Grimaldi, R., Osuna-Cabello, M., Norval, S., Riley, J., Stojanovski, L., Simeons, F. R. C., Wyatt, P. G., Delves, M. J., Meister, S., Duffy, S., Avery, V. M., Winzeler, E. A., Sinden, R. E., Wittlin, S., Gray, D. W., … Gilbert, I. H. (2016). Discovery of a quinoline-4-carboxamide derivative with a novel mechanism of action, multistage antimalarial activity, and potent in vivo efficacy. Journal of Medicinal Chemistry, 59(21), 9672–15. https://doi.org/10.1021/acs.jmedchem.6b00723
- Bowe, C. L., Mokhtarzadeh, L., Venkatesan, P., Babu, S., Axelrod, H. R., Sofia, M. J., Kakarla, R., Chan, T. Y., Kim, J. S., Lee, H. J., Amidon, G. L., Choe, S. Y., Walker, S., & Kahne, D. (1997). Design of compounds that increase the absorption of polar molecules. Proceedings of the National Academy of Sciences of the United States of America, 94(22), 12218–12223. https://doi.org/10.1073/pnas.94.22.12218
- Cancer_Genome_Atlas_Network. (2012). Comprehensive molecular portraits of human breast tumors. Nature.
- Di, L., Kerns, E. H., & Carter, G. T. (2009). Drug-like property concepts in pharmaceutical design. Current Pharmaceutical Design, 15(19), 2184–2194. https://doi.org/10.2174/138161209788682479
- Flynn, E. (2007). Pharmacokinetic Parameters. In S. J. Enna & D. B. Bylund (Eds.), xPharm: The comprehensive pharmacology reference. Elsevier.
- Freshney, R. I. (2002). Cell line provenance. Cytotechnology, 39(2), 55–67. https://doi.org/10.1023/A:1022949730029
- Ge, L., & Mordiffi, S. Z. (2017). Factors associated with higher caregiver burden among family caregivers of elderly cancer patients: A systematic review. Cancer Nursing, 40(6), 471–478. https://doi.org/10.1097/NCC.0000000000000445
- Haggag, M. W., Abou-El-Ella, S. M., & Abouziena, H. F. (2017). Phytochemical analysis, antifungal, antimicrobial activities and application of eichhornia crassipes against some plant pathogens. Planta Daninha, 35(e017159560), 1-11. doi: 10.1590/s0100-83582017350100026
- Hu, F.-Q., Liu, L.-N., Du, Y.-Z., & Yuan, H. (2009). Synthesis and antitumor activity of doxorubicin conjugated stearic acid-g-chitosan oligosaccharide polymeric micelles. Biomaterials, 30(36), 6955–6963. https://doi.org/10.1016/j.biomaterials.2009.09.008
- Hutchinson, L., & Kirk, R. (2011). High drug attrition rates—where are we going wrong? Nature Reviews. Clinical Oncology, 8(4), 189–190. https://doi.org/10.1038/nrclinonc.2011.34
- Jo, H. Y., Kim, Y., Park, H. W., Moon, H. E., Bae, S., Kim, J., Kim, D. G., & Paek, S. H. (2015). The unreliability of MTT assay in the cytotoxic test of primary cultured glioblastoma cells. Experimental Neurobiology, 24(3), 235–245. https://doi.org/10.5607/en.2015.24.3.235
- Johansen, S., Cvancarova, M., & Ruland, C. (2017). The effect of cancer patients’ and their family caregivers’ physical and emotional symptoms on caregiver burden. Cancer Nursing, 41(2), 91-99. https://doi.org/10.1097/NCC.0000000000000493
- Karakaş, D., Ari, F., & Ulukaya, E. (2017). The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turkish Journal of Biology = Turk Biyoloji Dergisi, 41(6), 919–925. https://doi.org/10.3906/biy-1703-104
- Kern, E., & Di, L. (2008). Drug-like properties: Concepts, structure design and methods. Elsevier.
- Kumar, P., Nagarajan, A., & Uchil, P. D. (2018). Analysis of cell viability by the MTT assay. Cold Spring Harbor Protocols, 2018(6), pdb.prot095505. https://doi.org/10.1101/pdb.prot095505
- Kumar, S., Kumar, R., Dwivedi, A., & Pandey, A. K. (2014). In vitro antioxidant, antibacterial, and cytotoxic activity and in vivo effect of syngonium podophyllum and eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. BioMed Research International, 2014, 11, 1-11. https://doi.org/10.1155/2014/459452
- Makhafola, T. J., & McGaw, L. J. (2017). Chapter 3 - Burden and health policy of cancers in Africa. In V. Kuete (Ed.), Medicinal spices and vegetables from Africa (pp. 93–107). Academic Press.
- McMaster, M. C. (2007). HPLC, A practical user’s guide. Wiley.
- Moreno, L., & Pearson, A. D. J. (2013). How can attrition rates be reduced in cancer drug discovery? Expert Opinion on Drug Discovery, 8(4), 363–368. https://doi.org/10.1517/17460441.2013.768984
- Mtewa, A., Ngwira, K., Lampiao, F., Weisheit, A., & Tolo, C. J. J. D. R. D. (2018). Fundamental methods in drug permeability, Pka, LogP and LogDx determination. Journal of Drug Research and Development,4(2), 2470–1009.2146. DOI:10.16966/2470-1009.146
- Mtewa, A., Sesaazi, D. C., Lampiao, F.(2020). Structural and in silico characterization of small molecules isolated from Eichhornia crassipes. Evidence-Based Complementary and Alternative Medicine, 2020(Article ID 1375639), 1-11. https://doi.org/10.1155/2020/1375639
- Mtewa, A. G., Deyno, S., Ngwira, K., Lampiao, F., Peter, E. L., Ahovegbe, L. Y., … Sesaazi, D. C. (2018). Drug-like properties of anticancer molecules elucidated from Eichhornia crassipes. Journal of Pharmacognosy and Phytochemistry, 7(5), 2075–2079. https://www.phytojournal.com/archives/2018/vol7issue5/PartAJ/7-5-381-691.pdf
- Pan, S.-Y., Zhou, S.-F., Gao, S.-H., Yu, Z.-L., Zhang, S.-F., Tang, M.-K., … Ko, K.-M. (2013). New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evidence-Based Complementary and Alternative Medicine, 2013, 1-11. https://doi.org/10.1155/2013/627375
- Parkin, D. M. (2004). International variation. Oncogene, 23(38), 6329–6340. https://doi.org/10.1038/sj.onc.1207726
- Patrick, G. (2017). An introduction to medicinal chemistry (6th ed.). Oxford University Press.
- Rostron, C. (2020). Drug design and development. Oxford University Press.
- Schneider, G. (2013). Prediction of drug-like properties. In Madame curie bioscience database [Online]. Landes Bioscience, 2000-2013. https://www.ncbi.nlm.nih.gov/books/NBK6404/. Accessed 12th January, 2020.
- Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., … Boyd, M. R. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI: Journal of the National Cancer Institute, 82(13), 1107–1112. https://doi.org/10.1093/jnci/82.13.1107
- Stewart, B. W., Bray, F., Forman, D., Ohgaki, H., Straif, K., Ullrich, A., & Wild, C. P. (2015). Cancer prevention as part of precision medicine: ‘plenty to be done’. Carcinogenesis, 37(1), 3-9. https://doi.org/10.1093/carcin/bgv166
- Takebe, T., Imai, R., & Ono, S. (2018). The current status of drug discovery and development as originated in United States academia: the influence of industrial and academic collaboration on drug discovery and development. Clinical and Translational Science, 11(6), 597–606. https://doi.org/10.1111/cts.12577
- Thorn, C. F., Oshiro, C., Marsh, S., Hernandez-Boussard, T., McLeod, H., Klein, T. E., & Altman, R. B. (2011). Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenetics and Genomics, 21(7), 440–446. https://doi.org/10.1097/FPC.0b013e32833ffb56
- Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., & Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65(2), 87–108. https://doi.org/10.3322/caac.21262
- Tyagi, T., & Agarwal, M. (2017). Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. Journal of Pharmacognosy and Phytochemistry., 6(1), 195–206. doi: 10.22159/ijcpr.2017.v9i3.19970
- Xie, Y., Ding, Z., Duan, W., & Ye, Q. (2011). Isolation and purification of terpenoids from Celastrus aculeatus Merr. by high-speed counter-current chromatography. Journal of Medicinal Plants Research., 6(12), 2520–2525. https://doi.org/10.5897/JMPR11.513