ABSTRACT
Breast cancer was considered as a kind of prone breast tumors with the complicated pathological mechanisms and diverse clinical classifications. In the clinical treatments of HER2-positive tumor patients, HER2 monoclonal antibodies, such as Herceptin, have shown well-defined therapeutic effects. Nevertheless, due to the heterogeneity of breast cancers, drug resistance inevitably appeared during the application of Herceptin. In order to fully understand the immune tolerance status of the tumor microenvironment in the population of sensitive and insensitive patients, this study carried out a series of studies through Luminex cytokines assay, clinicopathological analysis, immunofluorescence, and PCR. The results confirmed that in clinical samples sensitive to Herceptin, there were a large number of macrophages, and the protein expression levels and in situ expression of macrophage-related chemokines and inflammatory mediators are significantly higher than drug-resistant tumor samples. Further studies found that T cell function has a low correlation with tumor growth, and there are obvious obstacles in the process of peripheral blood immune cells entering the tumor microenvironment. In summary, this study provided clues for understanding the clinical drug resistance of HER2 monoclonal antibody and the clinical rational use of drugs and combination drugs.
Introduction
According to the data report of the International Cancer Registry released by the World Health Organization (WHO) in 2020, there are about 2.3 million new breast cancer cases worldwide each year, accounting for 14% of all female cancer patients.Citation1,Citation2 Breast cancers, with an increasing incidence year by year, possess specific and distinct pathogenesis, heterogeneous prognoses, histological morphology, immunophenotypes, and treatment responses in patients, which serves as an indispensable element for global burden and trends by menopausal status.Citation2 Based on the results of molecular biology and gene sequencing researches, different and standard classification methods for breast cancer have emerged, such as gene chips, hormone effects, and molecular markers. Although there are still controversies in the classification of breast cancers, the current widely used standard for breast cancer is specified at the St. Gallen International Conference on Breast Cancer. Breast cancers can be divided into the following four categories: Luminal A, Luminal B, human epidermal growth factor receptor 2 (HER2)-positive, and triple-negative.Citation3–5 Breast cancers overexpressing HER2 are well-defined as high metastatic capacity and poor prognosis.Citation6
The molecular characteristics of HER2 positive type of breast cancer are HER2+, estrogen receptors (ER−), and progesterone receptors (PgR−), among which the proportion of HER2-positive breast cancer is often less than 10%.Citation3 Most HER2-positive breast cancers are invasive ductal carcinoma, and the pathological grade is mostly ranged from II to III.Citation3–5 Previous studies have shown that HER-2 gene mutations lead to the higher risk of HER2 phosphorylation and other signaling pathway abnormalities, which increase the clinical incidence of breast cancer.Citation6,Citation7 Accumulating studies have found that breast cancer patients with HER2 gene overexpression or mutations have the poor prognosis, most of which are advanced cases and prone to axillary lymph node metastasis.Citation7 Further investigations have showed that the distant metastasis of HER2-positive patients is far greater than other types, and the prognosis is the worst from the follow-up data and analysis of breast cancer patients.Citation8
In terms of clinical treatment, patients with high ECD (extracellular domain of HER-2) before treatment are more likely to tolerate endocrine therapy, suggesting that patients with high ECD are not sensitive to endocrine therapy.Citation9 Trastuzumab (Herceptin) is the first targeted therapy for HER-2, which can bind to the extracellular site of the tyrosinase receptor of the HER-2 protein of cancer cells, thereby inhibiting the growth of tumors.Citation10,Citation11 With the continuous deepening researches of HER-2, Pertuzumab, Lapatinib, and other drugs have also been widely used in the treatment of HER2-positive breast cancers, and treatment intent for HER2-positive breast cancer is being promoted.Citation12,Citation13 In addition, for the treatment of patients with advanced HER2-positive breast cancer, combination of Pertuzumab and Herceptin can achieve the dramatic tumor-free growth state,Citation12–14 whereas this therapeutic strategy is often combined with chemotherapy and is easy to result in drug resistance.Citation15,Citation16
Recently, the application of HER-2 monoclonal antibodies has greatly improved the pathological symptoms of patients with HER2-positive breast cancer and effectively improved disease-free survival.Citation15,Citation16 Studies have also shown that HER-2 monoclonal antibodies could reduce the rate of distant metastasis.Citation17,Citation18 Although anti-HER2 antibody therapy has brought unprecedented results to HER2-positive patients, serious drug resistance problems might arise during the period of application.Citation6,Citation16 The current researches mainly focus on the following aspects: firstly, the possible overexpression of HER2 family genes and their ligands might lead to drug resistance; secondly, when the structure of the HER2 protein changes for some reason, the binding affinity to the targeted drug will decrease; third, when the downstream pathway of HER2 is continuously activated and there is a staggering phenomenon between the downstream pathway of HER2 and other pathways, and the sensitivity of anti-HER2 treatments to the treatment of HER2-positive breast cancer will be reduced.Citation19–22 Therefore, it is still an important research direction to improve the effect of HER2-positive treatment and the efficacy of HER-2 monoclonal antibodies.
In the immune system, B cells and a variety of T cells will express HER-2; there are also quite a few studies on the relationship between the expression of HER-2 in immune cells and tumors.Citation23–25 So far, the research on the effect of HER-2 monoclonal antibody on the microenvironment of breast cancer tumors after long-term use has not been in-depth. In the present research, we obtained the HER2-positive breast cancer samples with the treatment of Herceptin, and demonstrated that macrophage chemotaxis contributes to the defective efficacy of Herceptin on the tumor-immune microenvironments in breast cancer patients.
Materials and methods
Human breast cancer samples collection
The clinical specimens from breast cancer patients were collected and subjected to further histological and biochemical analysis after obtaining informed written consent according to the approval of the Ethics Committee of Xinxiang Central Hospital, Xinxiang, China. All patients in this research were at least 18 y old, histologically diagnosed with a positive expression of HER2 (IHC score ≥3, or IHC score ≥3 and FISH +), and received surgery without chemotherapy or radiotherapy before surgery. Patients were treated with Herception for 6–12 months and then the tumor sections were obtained during surgery or diagnosis. The clinical stages of breast cancer were identified in line with the criteria of the American Journal of Critical Care (AJCC), as illustrated in Table S1.
Histopathological examination
Tumor tissue samples were fixed in formalin and embedded in paraffin. The tumor slices were then cut into 5 μm, dewaxed, and stained with hematoxylin and eosin (H&E) according to the standard protocol in-house. The representative images of H&E staining were obtained under an Olympus I × 73microscope (Tokyo, Japan).
Immunohistochemical assay
Paraffin-embedded tumor sections were dewaxed using the interactive incubation with dimethylbenzene, absolute ethyl alcohol, and H2O. Subsequently, the sections were blocked with 3% H2O2 and soaked in citrate buffer solution for heat-induced epitope retrieval. The prepared tissue slices were then incubated overnight at 4°C with the primary antibodies against Ki-67, HER2 (Abcam, Cambridge, MA, USA), followed by further incubation with streptavidin-horseradish peroxidase for additional 1 h. The positive staining of tumor tissues was visualized using the diaminobenzidine substrate and the representative images were acquired under the Olympus I × 73microscope.
Immunofluorescent staining
The tumor histological slices were dewaxed in an oven at 60°C for 2 h, deparaffinized in xylene for 3 times, and then immersed in anhydrous ethanol, 90% ethanol, 75% ethanol, distilled water, step-by-step. Subsequently, the slices were immersed in 10 mM sodium citrate buffer (pH 6.0) and the endogenous enzymes were removed in 3% H2O2 at room temperature for 10 min. The slices were then blocked with 5% BSA blocking solution at room temperature for 1 h and incubated with the primary antibodies against macrophage marker CD68 (Cat. No. 14-0688-82, Thermo Fisher Scientific, Waltham, MA, USA), IP-10, and IL-6 (Abcam), diluted in immunostaining primary antibody diluent (P0103, Beyotime, China), overnight at 4°C. The fluorescent areas were further visualized using the FITC anti-rabbit secondary antibodies and then counterstained with DAPI to visualize the nuclei (Abcam). The positive staining was obtained under the Leica TCS SPS microscope (Wetzlar, Germany).
Cytokine’s determination
Breast tumor tissues (10–50 mg) were weighed and homogenized in cold phosphate buffer saline (PBS) solution using ultrasonication. The mixture was then centrifuged at 12,000 rpm for 10 min and the supernatants were collected and subjected to further analysis. Total protein was quantified using Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). The cytokines in the tissue homogenates were determined using a ProcartaPlex Multiple Immunoassay kit on the Luminex 200 instrument (Merck Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Briefly, the magnetic beads were added into the assay plates and washed twice using the Wash Buffer under the Hand-Held Magnetic Plate Washer. Subsequently, the tissue homogenates and the standards were added and then incubated overnight at 4°C. The Detection Antibody Mixture and streptavidin-PE were added, respectively. Then the assay plates were pulsed in the Reading Buffer and determined on a Luminex 200 instrument. Cytokine concentrations were normalized as pg/mg tissue protein.
Blood immune cells analysis
Anticoagulant venous whole blood was collected and subjected to further analysis of immune cells using the automatic blood cell analyzer (BC-6600Plus, Mindray, Shenzhen, China) in Xinxiang Central Hospital. The percentage of leukocytes, lymphocytes, monocytes, neutrophils, basophils, and eosinophils was counted by fluorometric analysis.
Quantitative reverse transcription PCR
Total RNA from human tumor tissues was extracted using the RNAsimple Total RNA Kit (Tiangen, Beijing, China) according to the manufacturer’s instruction. The tissues were homogenized in the TRIzol solution (Tiangen). The concentration of total RNA was determined using NanoDrop Microvolume UV-Vis Spectrophotometer (Thermo Fisher Scientific) and subsequently reverse transcribed into cDNA (500 ng per unit) using Hifair™ cDNA Synthesis Kit (Yeasen, Shanghai) with consecutive incubation of 25°C for 5 min, 42°C for 30 min. and 85°C for 5 min. Quantitative real-time PCR was performed with the cDNA and the specific primers using SYBRⓇ Green Realtime PCR Master Mix (Yeasen) by 7500 Fast Real-Time PCR System (Applied Biosystems, Foster city, CA, USA) for PCR amplification with holding stage (95°C for 30 s), cycling stage (95°C for 15 s and 60°C for 34 s for 40 cycles). GAPDH was used as the internal housekeeping gene for human samples. The sequences of used primers were listed in Table S2.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (Version 8.0, La Jolla, CA, USA). Differences between two groups were conducted by unpaired two-tailed Student’s t-test. Pearson correlation was performed to evaluate the relationship between two elements. All data were presented as mean ± standard error of the mean (SEM). Differences were considered as statistically significant when p value was less than 0.05.
Results
Defective efficacy of herceptin occurred in the clinical treatment of HER2-positive breast cancer
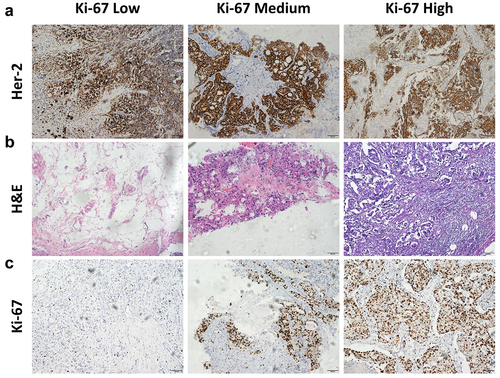
Given the classification of breast cancer, patients overexpressing HER2 represent as a certain number of clinical incidences. Despite the therapeutic capacity of humanized monoclonal antibodies toward HER2, the drug resistance frequently and inevitably appeared, which could be identified in our clinical cases from Xinxiang Central Hospital. To illustrate the potential mechanism of defective efficacy, 11 patients with the overexpression of HER2 and receiving Herceptin treatment were included in the present research (). According to the histopathological examinations of breast cancer biopsies, there indeed existed drug insensitivity in clinical (). Compared to the Herceptin-responsive individuals, more tumor foci, less accumulation of immune cells, and higher expression of Ki-67 were observed in patients suffering from Herceptin-disability ().
Figure 1. The pathological features for HER2-positive breast cancer patients. (a) Immunohistopathological staining of HER2 of the breast cancer sections (scale bar, 100 μm). (b) H&E staining of the breast cancer sections (scale bar, 100 μm). (c) Immunohistopathological staining of ki-67 of the breast cancer sections (scale bar, 100 μm). n = 11; data were presented as the representative images.
Macrophage hypofunction predicted a low herceptin responsivity in HER2-positive breast cancer
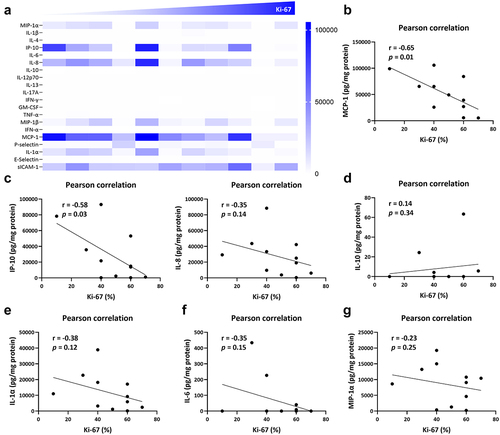
To fully characterize the regulatory mechanism of Herceptin-elicited immune responses in breast cancer microenvironment, several biochemical and immunophenotyping investigations were performed in the present research. As shown in by Luminex assay, a distinct cytokine, chemokine, and adhesive molecule profile was defined in line with the expression of Ki-67 from low to high expression. Meanwhile, the protein level of macrophage inflammatory protein-1 alpha (MIP-1α), C-X-C motif chemokine ligand 10 (CXCL10, also known as IP-10), IL-8, monocyte chemoattractant protein-1 (MCP-1), and IL-1α was decreased along with increased level of Ki-67 (). Further correlation analyses demonstrated that the level of MCP-1 was significantly and negatively related to the expression of Ki-67 (). The similar mode was observed in the relationship between Ki-67 and IP-10, or IL-8 (), which were mainly derived from tissue-resident or newly-recruited macrophages.Citation26,Citation27 In addition, we also focused on the cytokines release from macrophages and revealed that there seemed an upregulation of IL-10 in Ki-67-dominant tumor microenvironment, indicating the increased infiltration of macrophages in Herceptin-defective breast cancer (). However, the inflammatory mediators, IL-1α, IL-6, and MIP-1α were negatively associated with the level of breast cancer proliferation (). In brief, disturbed function of macrophages might contribute to the immune tolerance of Herceptin in breast cancer microenvironment.
Figure 2. Macrophage hypofunction was closely associated with the efficacy of herceptin on breast cancer. (a) Protein level of inflammatory mediators in the tissue homogenates of breast cancer biopsies, including cytokines, chemokines, and adhesive molecules. (b) The correlation between the percentage of ki-67 and the protein level of MCP-1. (c) The correlation between the percentage of ki-67 and the protein level of IP-10 and IL-8. (d) The correlation between the percentage of ki-67 and the protein level of IL-10. (e) The correlation between the percentage of ki-67 and the protein level of IL-1α. (f) The correlation between the percentage of ki-67 and the protein level of IL-6. (g) The correlation between the percentage of ki-67 and the protein level of MIP-1α. Pearson correlation was performed using GraphPad prism. n = 11; p < .05 was considered statistically significant.
T cell function exerted a relatively little effect on Herceptin efficacy in breast cancer patients
Furthermore, tumor necrosis factor alpha (TNF-α) was also subjected to the correlation analysis with Ki-67; However, there remained little relationship between TNF-α and Ki-67 (Fig. S1A). In addition to the infiltration and function of macrophages, T cell function was studied in the present research, including IFN-γ from T helper 1 (Th1) cells, IL-13 from Th2 cells, IL-17A from Th17 cells.Citation28 As illustrated in Fig. S1B-D, relatively low relationship between IFN-γ, IL-13, IL-17A, and Ki-67 was observed, mainly referring to the fact that T cell function might serve as a negligible effect on Herceptin-defective HER2-positive breast cancer patients.
The tissue-residence and function of macrophages was closely associated with the effects of herceptin on breast cancer
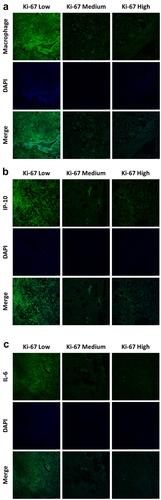
In view of the above findings, we mainly focused on the tissue-residence and function of macrophages in HER2-positve breast cancer. The survival rate analysis of breast cancer patients in TCGA database suggested that higher expression of CD68 displayed higher survival probability of breast cancer patients (). Moreover, in the Herceptin-sensitive specimens, a large population of macrophages were observed in the local tissue of breast cancer and with the increased expression of Ki-67, tissue-resident macrophages were dramatically decreased (). Moreover, the tissue expression of macrophage-derived inflammatory mediators was further identified and suggested that in line with the results in , the in-situ expression of IP-10 and IL-6 was largely scattered around the location of tissue-resident macrophages and passively related to the level of Ki-67 (), which were consistent with the results from and collectively indicated that the cytokines release property of macrophages might account for the sensitivity of Herceptin in breast cancer.
Figure 3. The relationship between macrophages and the survival rate of breast cancer patients. (a) The survival analysis of CD68 in the database of breast cancer in TCGA. (b) The correlation between the CD68 and CXCL-10 (IP-10). (c) The correlation between the CD68 and CCL2 (MCP1). (d) The correlation between the CD68 and IL-6.
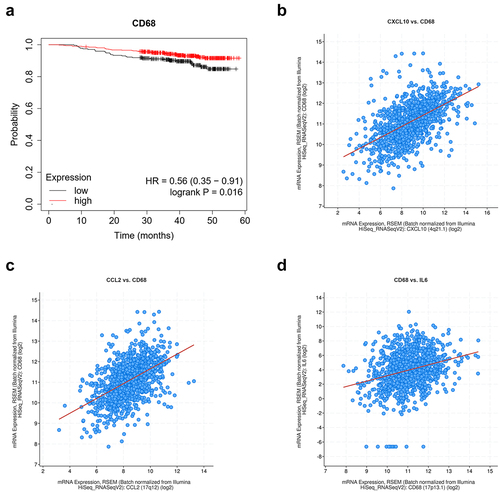
Figure 4. The population and function of macrophages was closely associated with the effects of herceptin on breast cancer. (a) Immunofluorescent staining of macrophages on the tissue sections of breast cancers (scale bar, 50 μm). (b) Immunofluorescent staining of IP-10 on the tissue sections of breast cancers (scale bar, 50 μm). (c) Immunofluorescent staining of IL-6 on the tissue sections of breast cancers (scale bar, 50 μm). Data were presented as the representative images of immunofluorescent staining.
The population of immune cells in peripheral blood merely contributed to the efficacy of herceptin on breast cancer
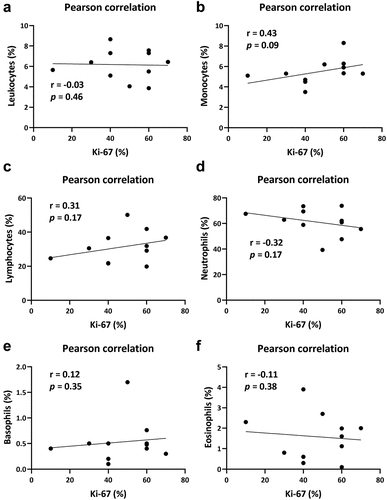
To further confirm the source of immune cells in tumor microenvironment, peripheral blood was obtained and subjected to further analysis by automatic blood cell analyzer. As illustrated in , there was little significance between Ki-67 and leukocytes. Further identification of subpopulation of immune cells showed that the expression of Ki-67 was positively related to the population of monocytes (), lymphocytes (), and basophils (), whereas, negatively related to the population of neutrophils (), and eosinophils (). Nevertheless, no statistical significance was observed between Ki-67 and the population of immune cells (). Taken together, though there remain a positive correlation between monocytes and Ki-67, disability of chemotaxis of monocytes or lymphocytes might exist in Herceptin-defective breast cancer patients.
Figure 5. The population of immune cells in peripheral blood merely contributed to the efficacy of herceptin on breast cancer. (a) The correlation between the percentage of ki-67 and the population of leukocytes. (b) The correlation between the percentage of ki-67 and the population of monocytes. (c) The correlation between the percentage of ki-67 and the population of lymphocytes. (d) The correlation between the percentage of ki-67 and the population of neutrophils. (e) The correlation between the percentage of ki-67 and the population of basophils. (f) The correlation between the percentage of ki-67 and the population of eosinophils. Pearson correlation was performed using GraphPad prism. n = 11; p < .05 was considered statistically significant.
The mRNA expression of chemokines and inflammatory cytokines from macrophages was observed in herceptin-defective HER2-positive breast cancer
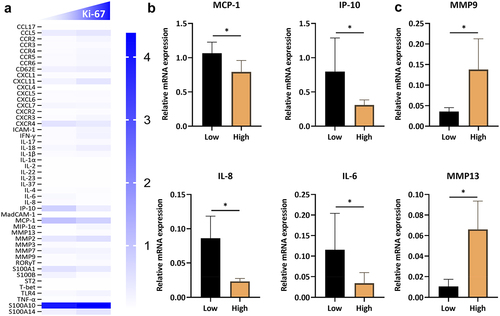
In order to uncover the critical role of macrophages in drug sensitivity in breast cancer, mRNA expression profile of macrophages was conducted in the present research. From the heatmap in , we analyzed the mRNA level of several chemokines, adhesive molecules, cytokines, alarmins, S100 proteins, and matrix metalloproteinases (MMPs). By contrast to the Herceptin-insensitive specimens, higher expression of MCP-1, IP-10, IL-8, and IL-6 was identified in Herceptin-sensitive breast cancer patients (), which were closely consistent with the protein level in . Additionally, increased expression of MMP9 and MMP13 was observed in patients expressing a high level of Ki-67 (), which might be correlated with the poor prognoses and invasion of breast cancer.Citation29,Citation30
Figure 6. The mRNA expression of several inflammatory mediators in herceptin-defective HER2-positive breast cancer. (c) The mRNA expression profile of inflammatory mediators in the tissue homogenates of breast cancers. (b) The mRNA expression level of MCP-1, IP-10, IL-8, and IL-6 in the ki-67 low and high expression pattern of breast cancer patients. (c) The mRNA expression level of MMP9 and MMP13 in the ki-67 low and high expression pattern of breast cancer patients. Data are presented as mean ± SEM. p < .05 was considered statistically significant.
The mRNA expression of chemokine receptors and S100 proteins negligibly participated in the sensitivity of herceptin in breast cancer
To confirm the role of macrophages chemotaxis in drug efficacy in breast cancer, further investigations focused on the mRNA expression level of chemokine receptors. The results in Fig. S2A suggested that there seemed an increased tendency of chemokine receptors, including CXCR2, CCR2, CCR3, CCR4, CCR5, and CCR6, in Herceptin-insensitive breast cancer patients, which largely referred to the dysfunction of gene transcripts of breast cancer. In addition, similar results were also confirmed in the expression of S100 proteins, such as S100A7, S100A8, and S100A14 (Fig. S2B). Briefly, the present research posted a potential mechanism of Herceptin-efficacy in breast cancer and suggested that macrophage chemotaxis played a vital role in the efficacy of Herceptin on the tumor-immune microenvironments.
Discussion
Breast cancer, as a type of malignant tumor that occurs frequently in women, has a serious impact on the quality of life and survival of patients. With the change of people’s living habits and the exposure of exogenous tumor-causing factors, the incidence of breast cancer is increasing year by year.Citation1,Citation2 The mechanism of breast cancer is relatively complicated, involving gene mutations, hormonal disorder, abnormal signal transduction, external pressure, and other factors.Citation31–33 The clinical heterogeneity and prevalence mutations of breast cancers lead to its diverse disease classifications and therapeutic options.Citation34,Citation35 Based on its clinical symptoms and differences in protein expression pattern, breast cancer is currently divided into Luminal A, Luminal B, HER2-positive, and triple-negative types, of which, the incidence of HER2-positive breast cancer is about 10%–30%.Citation4,Citation5,Citation34 In response to the high expression of HER2 and the abnormal downstream signal transduction, researchers have developed several monoclonal antibodies and small-molecule drugs targeting HER2, which could significantly alleviate the disease progress of breast cancer patients and inhibit tumor cell growth and metabolism.Citation15,Citation17 However, in the course of long-term use, drug resistance inevitably appeared, leading to an increase in the mortality rate of breast cancer patients.Citation6,Citation13,Citation22 In order to explore the characteristics of the tumor immune microenvironment of breast cancer patients, the present study included 11 HER2-positive patients from Xinxiang Central Hospital. Relying on the pathological and therapeutic properties, there are sensitive and drug-defective individuals. From the level of genes, proteins, and in situ expression, macrophage chemotaxis might play an important role in the treatment of breast cancer by Herceptin.
As a type of epidermal growth factor highly expressed in tumor cells, HER2 mainly mediates the survival, expansion, and distant metastasis of tumor cells, thereby inducing tumor occurrence and development through dimerization of the receptor and the subsequent autophosphorylation of tyrosine residues within the cytoplasmic domain.Citation36,Citation37 Overexpression of HER2 serves as a prognostic and predictive biomarker in several breast cancers, gastroesophageal cancers, ovary cancers, and lung cancers.Citation36,Citation37 The monoclonal antibody targeting HER2 could neutralize the biological and pathological effects of HER2, inhibit the growth of tumor cells, and improve the five-year survival rate and life quality of patients.Citation38,Citation39 As the first monoclonal antibody to treat HER2-positive breast cancer, Herceptin has a significant therapeutic effect in the course of clinical treatment.Citation10,Citation15 However, resistance inevitably appeared, as shown in . Therefore, it is of great significance to explore the efficacy mechanism of Herceptin.
In addition to tumor cells, macrophages, dendritic cells (DCs), CD4+ T cells, CD8+ T cells (cytotoxic T lymphocytes, CTLs), natural killer cells (NK cells) and B cells together constitute the tumor immune microenvironment.Citation40,Citation41 Upon dysfunction of immune cells or tumor cell overgrowth, the expression level of the key molecules that inhibit tumor cell growth was abnormal, loses the function of suppressing tumors, and then leads to the occurrence of tumor.Citation40 In addition to being expressed on tumor cells, HER2 protein also has a certain level of expression on T cells and other immune cells.Citation25 Targeted inhibition of HER2, while restricting tumor growth, might cause immune tolerance or low immune function of the tumor microenvironment.Citation42 Herein, the present research aimed to use biochemical and immunological methods to explore the effects of Herceptin on the tumor immune microenvironment and provide research ideas for its drug efficacy mechanism. The study first explored the expression of inflammatory mediators, chemokines, adhesion molecules, etc. derived from immune cells, and confirmed that the expression level of the chemokine, MCP-1, in Herceptin-sensitive individuals was significantly higher than in Herceptin-defective individuals (). MCP-1 is mainly derived from monocytes, such as macrophages, and can recruit macrophages to infiltrate the local area of breast cancers.Citation43 Furthermore, we also found that the expression levels of chemokines derived from macrophages, such as IP-10 and IL-8, were negatively correlated with tumor growth (). The results of in situ expression further confirmed that there were a large number of macrophages in Herceptin-sensitive individuals (), not T cell function (Figure S1), which collectively suggested that macrophages played a key role in the application of Herceptin.
To further clarify the source of macrophages recruited in the breast cancer tumor microenvironment, the following study focused on the population of immune cells in the peripheral blood of patients, which shown that there was no significant correlation between the total number of leukocytes and the growth of tumor cells. Further analysis showed that the number of peripheral monocytes and lymphocytes were positively correlated with tumor growth (), which meant that the number of monocytes and lymphocytes in the peripheral blood of Herceptin-sensitive individuals was relatively low; the opposite phenomenon was reflected in the peripheral blood of Herceptin-defective individuals (). This phenomenon might be attributed to the disability of peripheral immune cells to infiltrate to the tumor microenvironment, which was further confirmed by the low protein and gene expression levels of chemokines in tumor tissues of tumor-defective individuals (). Moreover, the gene expression levels in tumor tissues showed that, compared with Herceptin-defective individuals, the expression levels of chemokine receptors in the tumor tissues of sensitive individuals were relatively low (), which further revealed that in tumor-defective individuals, though the expression level of chemokine receptors was high, their transcript translation function might be impaired, leading to the occurrence of drug efficacy.
In summary, the present research confirmed that the expression of macrophage-related chemokines and inflammatory mediators in clinical samples sensitive to Herceptin are significantly higher than drug-defective tumor samples. Further studies found that T cell function and the population of peripheral blood immune cells exerted a low correlation with tumor growth. Collectively, this study initially explored the critical role of macrophage chemotaxis in the tumor-immune microenvironment, and provided clues for understanding the clinical drug efficacy of HER2 monoclonal antibody and the clinical rational use of drugs and combination drugs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributions
Heng Li and Yu Song designed the research and wrote the manuscript; Yu Song, Qiao-chen Geng, Wen-jing An, Fu-cheng Zhang, Ran Jiang, Rui-sheng Zhao, Zhi-jian Deng and Heng Li performed the experiments. All the authors approved the final draft.
Consent to publish
Informed consent to publish the results was obtained from all participants included in the study.
Revised Supplementary Information.docx
Download MS Word (1.7 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/23723556.2024.2309715
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–11. doi:10.3322/caac.21660.
- Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Global Health. 2020;8(8):e1027–e1037. doi:10.1016/S2214-109X(20)30215-1.
- Eliyatkın N, Yalçın E, Zengel B, Aktaş S, Vardar E. Molecular classification of breast carcinoma: from traditional, old-fashioned way to a new age, and a new way. J Breast Health. 2015;11(2):59–66. doi:10.5152/tjbh.2015.1669.
- Zubair M, Wang S, Ali N. Advanced approaches to breast cancer classification and diagnosis. Front Pharmacol. 2021;11(2487). doi:10.3389/fphar.2020.632079.
- Viale G. The current state of breast cancer classification. Ann Oncol. 2012;23:x207–x210. doi:10.1093/annonc/mds326.
- Zheng G, Guo Z, Li W, Xi W, Zuo B, Zhang R, Wen W, Yang AG, Jia L. Interaction between HLA-G and NK cell receptor KIR2DL4 orchestrates HER2-positive breast cancer efficacy to trastuzumab. Signal Transduct Target Ther. 2021;6(1):236. doi:10.1038/s41392-021-00629-w.
- Sun P, Zhang X, Wang RJ, Ma QY, Xu L, Wang Y, Liao HP, Wang HL, Hu LD, Kong X. et al. PI3Kα inhibitor CYH33 triggers antitumor immunity in murine breast cancer by activating CD8 + T cells and promoting fatty acid metabolism. J Immunother Cancer. 2021;9(8):e003093. doi:10.1136/jitc-2021-003093.
- Wang H, Zhang C, Zhang J, Kong L, Zhu H, Yu J. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: a SEER based study. Oncotarget. 2017;8(16):26368–26379. doi:10.18632/oncotarget.14300.
- Fan W, Chang J, Fu P. Endocrine therapy efficacy in breast cancer: current status, possible mechanisms and overcoming strategies. Future Med Chem. 2015;7(12):1511–1519. doi:10.4155/fmc.15.93.
- Vu T, Claret FX. Trastuzumab: updated mechanisms of action and efficacy in breast cancer. Front Oncol. 2012;2:62. doi:10.3389/fonc.2012.00062.
- Nahta R, Esteva FJ. Herceptin: mechanisms of action and efficacy. Cancer Lett. 2006;232(2):123–138. doi:10.1016/j.canlet.2005.01.041.
- Patel TA, Ensor JE, Creamer SL, Boone T, Rodriguez AA, Niravath PA, Darcourt JG, Meisel JL, Li X, Zhao J. et al. A randomized, controlled phase II trial of neoadjuvant ado-trastuzumab emtansine, lapatinib, and nab-paclitaxel versus trastuzumab, pertuzumab, and paclitaxel in HER2-positive breast cancer (TEAL study). Breast Cancer Res. 2019;21(1):100. doi:10.1186/s13058-019-1186-0.
- Leung W-y, Roxanis I, Sheldon H, Buffa FM, Li J-L, Harris AL, Kong A. Combining lapatinib and pertuzumab to overcome lapatinib efficacy due to NRG1-mediated signalling in HER2-amplified breast cancer. Oncotarget. 2015;6(8):5678–5694. doi:10.18632/oncotarget.3296.
- Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal transduction and targeted therapy. 2019;4(1):34. doi:10.1038/s41392-019-0069-2.
- Maadi H, Soheilifar MH, Choi WS, Moshtaghian A, Wang Z. Trastuzumab mechanism of action; 20 years of research to unravel a dilemma. Cancers Basel. 2021;13(14):3540. doi:10.3390/cancers13143540.
- Hunter FW, Barker HR, Lipert B, Rothé F, Gebhart G, Piccart-Gebhart MJ, Sotiriou C, Jamieson SMF. Mechanisms of efficacy to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer. 2020;122(5):603–612. doi:10.1038/s41416-019-0635-y.
- Altunay B, Morgenroth A, Beheshti M, Vogg A, Wong NCL, Ting HH, Biersack H-J, Stickeler E, Mottaghy FM. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging. 2021;48(5):1371–1389. doi:10.1007/s00259-020-05094-1.
- Wang J, Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal transduction and targeted therapy. Signal Transduct Target Ther. 2019;4(1):34–34. doi:10.1038/s41392-019-0069-2.
- Pernas S, Tolaney SM. HER2-positive breast cancer: new therapeutic frontiers and overcoming efficacy. Ther Adv Med Oncol. 2019;11:1758835919833519. doi:10.1177/1758835919833519.
- Gajria D, Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab efficacy and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11(2):263–275. doi:10.1586/era.10.226.
- Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146(3):264–275. doi:10.1016/j.jconrel.2010.04.009.
- Pohlmann PR, Mayer IA, Mernaugh R. Efficacy to trastuzumab in breast cancer. Clin Cancer Res. 2009;15(24):7491. doi:10.1158/1078-0432.CCR-09-0636.
- Jeon I, Lee JM, Shin KS, Kang T, Park MH, Seo H, Song B, Koh CH, Choi J, Shin YK. et al. Enhanced immunogenicity of engineered HER2 antigens potentiates antitumor immune responses. Vaccines (Basel). 2020;8(3):403. doi:10.3390/vaccines8030403.
- Griguolo G, Pascual T, Dieci MV, Guarneri V, Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J Immunother Cancer. 2019;7(1):90. doi:10.1186/s40425-019-0548-6.
- Lin X-L, Wang X-L, Ma B, Jia J, Yan Y, Di L-J, Yuan Y-H, Wan F-L, Lu Y-L, Liang X. et al. HER2-specific T lymphocytes kill both trastuzumab-defective and trastuzumab-sensitive breast cell lines in vitro. Chin J Cancer Res. 2012;24(2):143–150. doi:10.1007/s11670-012-0143-6.
- Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F, Ren X, Yu J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (review). Int J Oncol. 2016;48(1):5–12. doi:10.3892/ijo.2015.3234.
- Devaraj S, Jialal I. Increased secretion of IP-10 from monocytes under hyperglycemia is via the TLR2 and TLR4 pathway. Cytokine. 2009;47(1):6–10. doi:10.1016/j.cyto.2009.02.004.
- Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi:10.1016/j.cyto.2014.09.011.
- Kim G-E, Lee JS, Choi Y-D, Lee K-H, Lee JH, Nam JH, Choi C, Kim SS, Park MH, Yoon JH. et al. Expression of matrix metalloproteinases and their inhibitors in different immunohistochemical-based molecular subtypes of breast cancer. BMC Cancer. 2014;14(1):959. doi:10.1186/1471-2407-14-959.
- Zhang B, Cao X, Liu Y, Cao W, Zhang F, Zhang S, Li H, Ning L, Fu L, Niu Y. et al. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer. 2008;8(1):83. doi:10.1186/1471-2407-8-83.
- Tungsukruthai S, Petpiroon N, Chanvorachote P. Molecular mechanisms of breast cancer metastasis and potential anti-metastatic compounds. Anticancer Res. 2018;38:2607.
- Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W. et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106. doi:10.1016/j.gendis.2018.05.001.
- Imyanitov EN, Hanson KP. Mechanisms of breast cancer. Drug discovery Today: disease mechanisms. Drug Discov Today Dis Mech. 2004;1(2):235–245. doi:10.1016/j.ddmec.2004.09.002.
- Turashvili G, Brogi E. Tumor heterogeneity in breast cancer. Front Med. 2017;4:227. doi:10.3389/fmed.2017.00227.
- Rivenbark AG, O’Connor SM, Coleman WB. Molecular and cellular heterogeneity in breast cancer: challenges for personalized medicine. Am J Pathol. 2013;183(4):1113–1124. doi:10.1016/j.ajpath.2013.08.002.
- Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi:10.1155/2014/852748.
- English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol Diagn Ther. 2013;17(2):85–99. doi:10.1007/s40291-013-0024-9.
- Yu S, Liu Q, Han X, Qin S, Zhao W, Li A, Wu K. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6(1):31. doi:10.1186/s40164-017-0091-4.
- Di Modica M, Tagliabue E, Triulzi T. Predicting the efficacy of HER2-targeted therapies: a look at the host. Dis Markers. 2017;2017:7849108. doi:10.1155/2017/7849108.
- Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The crosstalk between tumor cells and the immune microenvironment in breast cancer. Implications Immunother. 2021;11(289). doi:10.3389/fonc.2021.610303.
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi:10.1038/s41591-018-0014-x.
- Steven A, Seliger B. The role of immune escape and immune cell infiltration in breast cancer. Breast Care. 2018;13(1):16–21. doi:10.1159/000486585.
- Dehqanzada ZA, Storrer CE, Hueman MT, Foley RJ, Harris KA, Jama YH, Kao T-C, Shriver CD, Ponniah S, Peoples GE. Correlations between serum monocyte chemotactic protein-1 levels, clinical prognostic factors, and HER-2/neu vaccine-related immunity in breast cancer patients. Clin Cancer Res. 2006;12(2):478. doi:10.1158/1078-0432.CCR-05-1425.

