ABSTRACT
The Africa Centres for Disease Control and Prevention Biosafety and Biosecurity Initiative being implemented across the 55 African Union Member States is presented. Based on consultations with Member States between 2019 and 2021, national-level capacity gaps were identified which informed the biosafety and biosecurity 5-year (2021–2025) strategic plan. The process of identifying national gaps, development, implementation and monitoring of the 5-year strategic plan is described. Notable achievements include development of a regional biosafety and biosecurity legislative framework now approved by African Union structures and process of domestication that has started in some countries; establishment and operationalisation of multi-sectoral regional biosafety and biosecurity technical working groups tasked with coordinating and monitoring implementation of the initiative, development and implementation of an accessible and regionally endorsed Regional Training and Certification Program for Biosafety and Biosecurity Professionals; establishment of a Regional Centre of Excellence for biosafety and biosecurity from where two cohorts of students in the two areas of biorisk management (16 students from 8 countries) and biological waste management (19 students from 10 countries) have been trained and development of a Regulatory and Certification Framework for Institutions Handling High-Risk Pathogens with three components of minimum standards of biosafety and biosecurity for high-containment facilities, a standard evaluation checklist for checking compliance and a certification framework for authorising performance of tasks related to dangerous pathogens. A guidance document with step-by-step process on how to translate regional successes to national implementation was developed and published. Based on notable regional successes of the initiative, Africa CDC co-chaired the Global Health Security Agenda Action Package 3 on Prevention and the Africa Signature Initiative Biosafety and Biosecurity Working Group. Challenges of delayed implementation due to the COVID-19 pandemic, limited resources to implement all planned activities and limited staff dedicated to biosafety and biosecuruty at Africa CDC.
Introduction
The 2016–2019 World Health Organization (WHO) Join External Evaluations (JEE) (World Health Organization Joint External Evaluations, Citation2019) and the 2019 and 2021 Global Health Security (GHS) Index reports (Global Health Security Index, Citation2019) both reported weakened biosafety and biosecurity systems among the Africa Union Member states. The 42 Africa Union Member States who participated in the WHO JEE had an average of 32% in Biosafety (World Health Organization Joint External Evaluations, Citation2019). Only two Member States scored 50% in Biosafety, and none surpassed the 30% score in the 2019 Global Health Security Index (Global Health Security Index Report, Citation2019) (.
Figure 1. Biosafety and Biosecurity Index scores of the 54 African countries included in the 2019 Global Health Security Index Report.
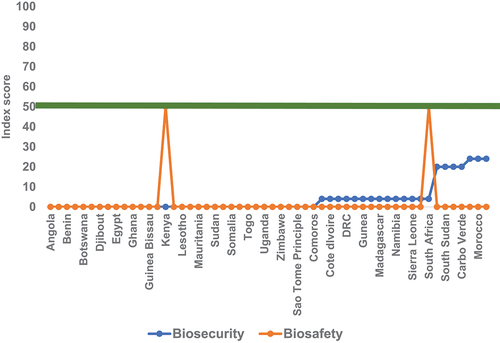
The main recommendation from the 2019 GHS Index report was that governments should include measurable biosecurity and biosafety benchmarks in national health security strategies and track progress on an annual basis. The WHO JEE also indicated limited capacity in biosafety, especially in the areas of coordination among different government and private sectors, lack of biosafety and biosecurity legislation, identification and management of high consequence agents and toxins, infrastructure, training and capacity building and financing.
Although the Africa Region responded well to the call to measure their preparedness capacities through JEE with 47 of the 55 (85%) African Union Member States having publicly published their reports, this has not been necessarily translated into tangible actions on the ground. As of June 2022, 34 (72%) of these 47 countries have completed their National Actions Plans for Health Security (WHO National Actions Plans for Health Security, Citation2022). The biggest challenge has been the lack of financing from the fiscus as part of pandemic preparedness, limited legislation that supports implementation of biosafety and biosecurity interventions at national level and unavailability of accessible biosafety and biosecurity focused training programmes that create adequate and appropriately trained and skilled biosafety and biosecurity experts.
There are several ways to address these gaps. The authors describe a regional approach to strengthening biosafety and biosecurity in Africa, led by the Africa Centres for Disease Control and Prevention (Africa CDC).
Methodology
A systematic approach was followed from (i) identification of overall goal of strengthening biosafety and biosecurity to (ii) identification of actual capacity gaps faced by Member States at implementation level, (iii) prioritisation and development of a regional strategy and (iv) implementation of the strategy.
Launch of the regional biosafety and biosecurity initiative – setting the overall goal
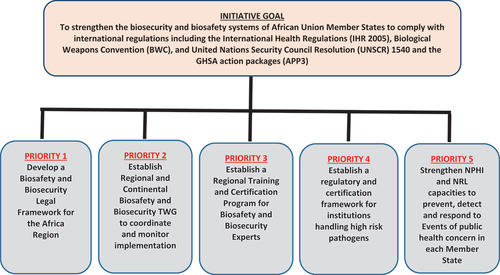
To ensure there is a focus and deliberate effort to strengthen biosafety and biosecurity, in May 2019, Africa CDC, in collaboration with Africa Union Member States and its regional and international partners, launched the Regional Biosafety and Biosecurity Initiative. The initiative’s goal is to strengthen the biosafety and biosecurity capacities of the Member States to enable them to comply with national and international requirements including the International Health Regulations of 2005, the Biological Weapons Convention, the United Nations Security Council Resolution 1540 and the multi-country Global Health Security Agenda (Africa CDC Biosafety and Biosecurity Initiative, Citation2019).
Among the areas prioritised under the initiative were to support the development and implementation of National Action Plans for Health Security (NAPHS), development of model legal frameworks and training and capacity building in biosafety and biosecurity.
Regional priorities – identification of actual capacity gaps faced by member states at national level
To understand the real problems that Member States were facing at national level, Africa CDC in collaboration with its five Regional Coordinating Centres held consultative meetings where capacity gaps and opportunities for improvement were discussed and agreed upon. Across the five regions, capacity gaps were identified in the areas of policies, legislation, training, identification of priority pathogens and establishments of coordination structures including regional, national technical working groups and associations (Africa CDC Biosafety and Biosecurity Initiative Report on the Consultative Process to Identify Priorities for Strengthening Biosafety and Biosecurity – Africa CDC, Citation2021b). summarises the areas identified by the five regions requiring strengthening.
Table 1. Regional priority areas for biosafety and biosecurity strengthening.
The 5-year strategic plan – prioritisation and development of a regional strategy
The capacity gaps identified by Member States were many and required a strategic approach to maximise the finite resources available. Hence, Africa CDC developed a Biosafety and Biosecurity Five Year Strategic Plan (2021–2021). The Strategic plan prioritised areas that aimed at building regional structures and systems that will smoothen implementation of biosafety and biosecurity at national level. Regional structures built using a consensus-based approach ensure alignment with national level needs creating opportunities for national level buy-in and smoothened domestication.
In addition, the 5-year strategic plan ensures a focused and coordinated implementation of the Biosafety and Biosecurity initiative in an environment with multiple stakeholders, including funders who can identify activities that align with their mandates in the region and proceed to implement these directly with Member States. Africa CDC will take the role of facilitating the engagement with countries while providing the overarching coordination role. The strategic plan identified five priority areas (Africa CDC Biosafety and Biosecurity Initiative 2021–2025 Strategic Plan – Africa CDC, Citation2021a). See .
Monitoring progress – development of a monitoring and evaluation framework
To track progress in the implementation of the regional strategy, a monitoring and evaluation framework was developed.
Implementation of the strategy – translation of regional strategy to national-level implementation
Global and regional measurement matrices including the GHS Index and WHO JEE measure capacities and capabilities at national level. Therefore, regional strategies require domestication in order to have an impact at national level. The Africa CDC developed a guidance document on how to translate the regional strategies to a national implementation (Africa CDC, Citation2022a). For example, the Regional Biosafety and Biosecurity Legislative Framework defines the regionally agreed scope of legislation required for effective implementation, which individual countries will then domesticate and align to national legislation. The strategy outlines a three-stage process, starting with the establishment of multisectoral National Biosafety and Biosecurity Technical Working Group that will coordinate implementation of the biosafety and biosecurity programme. The second step is development of a National Biosafety and Biosecurity Strategic Plan that is informed by a situation analysis and identification of priority areas which become the strategic objectives of the plan. Lastly, is the development of a monitoring and evaluation plan to track progress.
Results
Preliminary results between 2019 and 2022 are reported for each of the priority areas of the five-year strategic plan. summarises the outputs from the implementation of the priority areas of the Biosafety and Biosecurity Initiative.
Table 2. Summary of the biosafety and biosecurity initiative priority areas and the outputs for the period April 2019 to October 2022.
Regional biosafety and biosecurity legislative framework
As required by the Africa Union when developing regional documents, a full consultative and iterative process was followed in the development and adoption of the Regional Biosafety and Biosecurity Legislative Framework. Africa CDC coordinated consultation with Member States using its established five Regional Biosafety and Biosecurity Technical Working Groups (RBB-TWGs). About 44 of the 55 (80%) African Union Member States were represented in the 3-day review workshops where the draft framework was discussed. The adopted Legislative Framework was submitted to the Africa Union for review and adoption. The Legislative Framework was reviewed and approved by the Specialized Technical Committee (STC) for Health, Population and Drug Control and the STC Legal Affairs on it was to review and approval by the Council of Permanent Representatives, Executive Council and then by the Heads of States and Government by end of 2024.
The draft framework under review has the following domains: (i) authorisation of the establishment of a lead entity/agency responsible for regulating and managing biosafety and biosecurity systems, (ii) development of national standards for biosafety and biosecurity, (iii) authority for biological risk assessment, (iv) regulation of laboratory and facility requirements for handling High-Consequence Agents and Toxins (HCATs), (v) education, training and human resource development for all personnel who possess, use, manipulate, store, transfer or destroy/incinerate HCATs and (vi) transfer, storage and disposal of HCATs.
While the review process is ongoing, Africa CDC is coordinating the preparation of eventual domestication of the Regional Framework once endorsed. A pilot is planned for in three countries on how to conduct legal mapping and development of a country-specific domestication plan. One of these pilots was completed in Zambia in November 2022. Results of the pilot will be used to develop a regional guidance document on legal mapping and development of domestication plan that Member States can use to domesticate the Regional Legislative Framework.
Establishment and operationalisation of Regional Biosafety and Biosecurity Technical Working Groups (RBB-TWGs)
Five multisectoral Regional Biosafety and Biosecurity Technical Working Groups (RBB-TWGs) for the Central, East, North, Southern and West Africa regions were established and operationalised between November 2020 and March 2021. Official nominations were requested from Member States to constitute the multi-sectoral and multi-expert RBB-TWGs whose membership included human, animal, environmental health, security, customs, civil society, members of parliament, legal practitioners and institutions of high learning. Each RBB-TWG is led by a Chair and has defined Terms of Reference that includes its roles and responsibilities, membership, selection and term of office of the Chair, decision-making process, frequency, quorum and nature of meetings, among others.
The RBB-TWGs provided organised platforms for engaging Member States and reviewed and approved key policy and guidance documents including the Regional Biosafety and Biosecurity Legislative Framework, the Regulatory and Certification Framework for Institutions Handling High Risk Pathogens, the Regional Training and Certification Program for Biosafety and Biosecurity Professionals in the Africa Region. To date, three virtual meetings and one physical meetings per year were conducted for each of the RBB-TWG between November 2020 and October 2022.
Establish a Regional Training and Certification Program for Biosafety and Biosecurity Professionals (RTCP-BSBP)
Regional training and certification framework
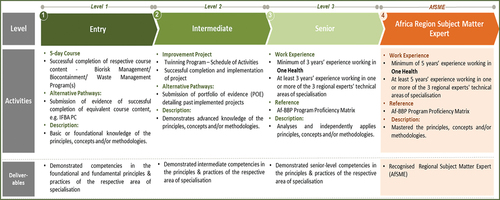
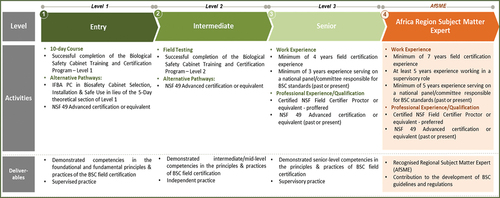
A Regional Training and Certification Framework for the Regional Training and Certification Program for Biosafety and Biosecurity Professionals (RTCP-BSBP) was developed, reviewed, endorsed by AU Member States and officially launched on 12 April 2022 at the African Union Headquarters (Africa CDC, Citation2022b). The framework describes the three competency levels of the four areas of specialisation of the RTCP-BSBP of (i) Biorisk Management, (ii) Biological Waste Management, (iii) Management of High-Containment Facilities (Biocontainment Engineering) ( and (iv) Installation, Certification, Servicing and Maintenance of Biological Safety Cabinets (.
The Examination and Certification Committee
An Examination and Certification Committee (ECC) responsible for independently monitoring the implementation of the certification programme in accordance with ISO 17024/2012 (General requirements for bodies operating certification of persons) was established (Africa CDC, Citation2022c). The 13 member ECC is constituted of competitively selected representatives of nine AU Member States (Ghana, Nigeria, Uganda, Kenya, Morocco, Cameroon, Zimbabwe, Egypt, Burundi), three Regional Institutions (African Society for Laboratory Medicine, Africa CDC, International Federation of Biosafety Association) and Masinde Muliro University of Science and Technology that offers degree program in biosafety and biosecurity. The roles and the responsibilities of the ECC include coordinating the review and updating of the RTCP-BSBSP framework and curriculum in line with current regional and international standards in biosafety and biosecurity, approving examinations and competency evaluations, reviewing portfolios submitted by trainees and recommending appropriate certification level, ensuring the African Union Register for Biosafety and Biosecurity professionals is up to date and shared widely and publicly.
Regional centre of excellence for biosafety and biosecurity
RTCP-BSBSP level 1 didactic trainings are conducted at designated Regional Centres of Excellence for Biosafety and Biosecurity (RCoEBB). In July 2022, a team of six experts from Namibia, Zimbabwe, Botswana, Lesotho and Africa CDC conducted an evaluation using a standard evaluation checklist approved by the RBB-TWGs and recommended the Regional Diagnostics and Demonstration Centre (RDDC) at the National Institute for Communicable Disease (NICD) South Africa as RCoEBB. The RDDC received is a certificate from Africa CDC confirming its designation as RCoEBB in July 2022.
Regional trainings for level 1 certification
In August 2022, two trainings were conducted in the specialised areas of Biorisk Management and Biological Waste Management. Sixteen participants from Botswana, Namibia, Lesotho, South Africa, eSwatini, Zambia and Mozambique successfully completed Level 1 certification in Biorisk Management. Nineteen participants from Angola, Botswana, Namibia, Lesotho, South Africa, eSwatini, Zambia and Mozambique and Zimbabwe successfully completed level 1 certification in Biological Waste Management.
Develop a regulatory and certification framework for institutions handling high-risk pathogens
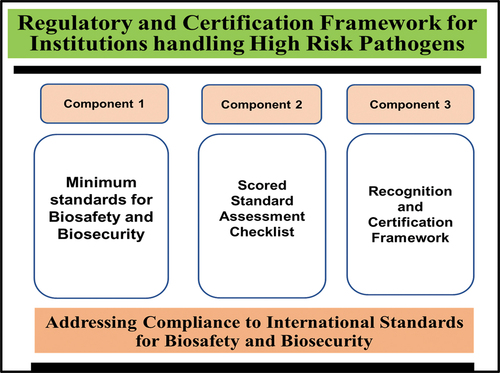
Despite the several epidemics in the Africa Region, there currently are no regionally accepted minimum standards for biosafety and biosecurity that AU Member States can enforce for compliance at institutions that are handling these epidemic-prone pathogens. In collaboration with AU Member States, Africa CDC coordinated the development, review and endorsement of the Regulatory and Certification Framework for Institutions Handling High Risk Pathogens for the Africa Region. The Framework officially launched at the African Union Headquarters in August 2022 has three components: (i) a set of minimum standards for biosafety and biosecurity that institutions handling high-risk pathogens must comply with, (ii) a standard evaluation checklist that AU Member States can use to check for continued compliance with the agreed minimum standards and (iii) a framework for certifying and authorising institutions that comply with the minimum standards to perform various activities with high-risk pathogens based on their compliance levels (Africa CDC). . The graduated certification level using a 0–5-star rating allows for incremental compliance with the set standards while recognising and rewarding current levels of compliance at each time of assessment.
Training of regional assessors
A total of 15 Regional Assessors for the Regulatory and Certification framework from Tanzania, Botswana, South Africa, Zambia, Malawi, Nigeria, Uganda and Kenya were trained. These certified assessors were enlisted on the African Union Register for Regional Assessors for the Regulatory and Certification Framework and will be deployed to assess applicant institutions and provide recommendations for appropriate star certification.
Training of trainers for implementers of the framework
About 21 implementors from 9 countries were trained as trainers of the Regulatory and Certification Framework. This is a method used for conducting Training of Trainers developed by Dr Gordon Pask (Citation1961). All the 21 participants were recommended as trainers and expected to lead implementation and training on requirements at national and facility level in-country.
Strengthening national public health institutes and national reference laboratories biosafety and biosecurity capacities
In response to the COVID-19 pandemic, Africa CDC developed a 6-day training of trainer’s programme on Biosafety and Biosecurity in the context of the COVID-19 pandemic. The rapid expansion in testing required appropriately trained and skilled testing personnel to minimise further spread of the infection among health personnel and communities. A total of 94 personnel were trained and certified as trainers in 11 countries of Cameroon, Congo, Democratic Republic of Congo, Eswatini, Kenya, Liberia, Niger, South Sudan, Uganda Zambia and Zimbabwe. Further step-down trainings led by these trainers were conducted in Kenya, Zambia and Zimbabwe.
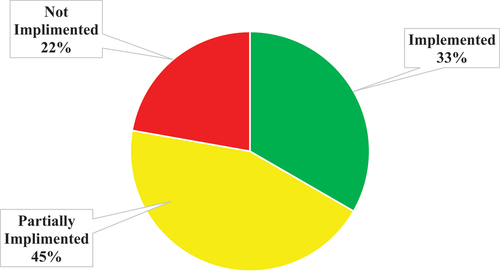
To date, 33% of the planned interventions in the strategic plan have been fully implemented, 45% partially implemented and 22% yet to be initiated (. Partially, mostly include rolling activities for the strategy period, e.g. three RCoEBB are planned for establishment by 2025 and one has been established already. Activities not yet started include those that funding has not yet been secured e.g. renovations and upgrades of institutions handling high risk pathogens. Other activities not yet started are those that depend on completion of other preceding activities, e.g. training on the BSBS Legislative Framework is only possible after completion of its review and adoption by the African Union.
Discussion
Biosafety and biosecurity capacities and capabilities of African Union Member States have long since been underdeveloped as reported by the global measurement matrices of the WHO JEE and GHS Index. To address this, innovative approaches are required, including taking regional approaches that can easily be translated to implementation at national level.
The Africa CDC Biosafety and Biosecurity Initiative Regional Biosafety seeks to build regional structures and systems that will enable Member States to implement national biosafety and biosecurity systems. Consensus at regional level allows for political buy-in at the highest level, enabling implementation at national level. For example, consensus by Member States at the African Union Level on the Biosafety and Biosecurity Legislative Framework provides leverage for technical people at national level to translate that commitment to implementation. As already being observed, a number of countries are adopting and domesticating the draft framework into their legislative reviews to ensure that it aligns with what the region has agreed upon. Similarly, endorsement of the Regional Training and Certification Framework for Biosafety and Biosecurity Professionals the Regulatory and Certification allows for acceptance of certified professionals across the continent. It also provided the basis upon which in-country trainings can now be standardised as was seen with other countries like Lesotho already endorsing it as their national training curriculum.
Translating these regional initiatives to national level requires a well-coordinated approach. The multi-sectoral Regional Biosafety and Biosecurity Technical Working Groups will be critical in proving the bridge between regional and national level efforts. Efforts are already underway to mirror this structure at national level with the establishment of National Biosafety and Biosecurity Technical Working Groups. In countries like Lesotho, Eswatini, Botswana, Mozambique, Uganda, Rwanda, Mali and Malawi, where national structures exist, these have began leading local domestication efforts. Consistency in engaging the regional technical working group is required to sustain transitioning from regional to national implementation.
As a regional institution, Africa CDC has limited staffing presents in-country, hence, there is a need for continued engagement of in-country partners who have continued presence on the ground to support domestication of regional initiatives. For example, Africa CDC has collaborated with in-country partners to jointly conduct step-down trainings in biosafety and biosecurity. Similarly, implementation of the Requirements of the Regulatory and Certification Framework and domestication of the Biosafety and Biosecurity Legislative Framework will require joint and close collaboration with in-country partners that operate at national level.
Further strengthening of Africa CDC Regional Coordinating Centres (RCCs) who are closer to Member States in their region will be critical to the success of translating regional to national implementation. Currently, of the five RCCs, the Central, Eastern and Southern are operational with Northern and Western with limited functionality. The Biosafety and Biosafety Programme has to date placed Biosafety and Biosecurity technical staff in the Southern and Western RCC with plans to place at the Central RCC by the end of 2022. Strengthened RCCs will provide a closer oversight role in implementation of biosafety and biosecurity at local level.
Availability of resources to support implementation is an anticipated challenge at both regional and national levels. Although Africa CDC has secured support for the first phase of its 5-year strategic plan, additional resources will be required to sustain and expand the programme. Continued lobbying for inclusion of biosafety and biosecurity into national plans and budgets is required. Some countries have taken this initiative, including Lesotho, who have developed a Biosafety and Biosecurity 5-year strategy as a national document to secure support from both the fiscus and implementing partners.
Conclusion
The Africa CDC Regional Biosafety and Biosecurity Initiative allows for consensus-building approach to identification of priorities and development of standardised regional interventions that can be domesticated at national level.
Author contribution
Talkmore Maruta – designed and implemented the initiative. Wrote the manuscript
Jaures Arnaud Noumedem Kenfack – implemented the initiative
Yenew Kebede Tebeje – provided technical coordination in the implementation of the initiative and reviewed the manuscript
Donewell Bangure – implemented the initiative
Ahmed E Ogwell Ouma – provided overall oversight of the initiate
Acknowledgments
The Africa CDC acknowledges the African Union Member States for availing their technical experts to participate in various activities of the initiative. Special thanks goes to the Global Affairs Canada’s Weapons Threat Reduction Program (GAC) that provided funding to implement the 5-year strategic plan and other various partners including the Nuclear Threat Initiative (NTI) and United States of America Centres for Disease Control and Prevention (US CDC) for providing technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Talkmore Maruta
Talkmore Maruta serves as the Acting Director of Programs at the African Society for Laboratory Medicine. He is a Public Health and Medical Laboratory professional with 22+ years of experience in the areas of laboratory systems strengthening, emergency preparedness and response and biosafety and biosecurity. Dr Maruta has worked with renowned institutions including Clinton Health Access Initiative (CHAI), Foundation for Innovative New Diagnostics (FIND), East Central and Southern Africa Health Community (ECSA-HC), Africa Centres for Disease Control and Prevention (Africa CDC) where he managed programs and projects across the Africa Region, the Caribbean and East Asia. His work in the region was recognized at the ASLM 2012 Conference with a ‘Distinguished Leadership” award and the “Best Employee” award in the World Bank supported Southern Africa TB Health Systems Strengthening (SATBHSS) project in 2019. Dr Talkmore Maruta PhD in Public Health, Masters in Public Health (MPH), Masters in Business Administration (MBA), Masters in International Affairs and Diplomacy BSc and a (Hons) degree in Medical Laboratory Sciences.
Jaures Arnaud Noumedem Kenfack
Jaures Arnaud Noumedem Kenfack is an experienced leader dedicated to improving health outcomes for disadvantaged populations. With expertise in laboratory operations, quality assurance, Biosafety and Biosecurity, HIV/AIDS, and health systems strengthening, he assesses performance, manages projects, and develops strategies. Dr. Noumedem's analytical mindset and communication skills drive innovative solutions. Holding a PhD in Clinical Biochemistry, he has over twelve years of experience in health projects. Since March 2021, he has served as a Biosafety and Biosecurity Technical Officer at Africa CDC. In this role, Dr. Noumedem supports African Union Member States in strengthening Biosafety and Biosecurity systems to comply with national, regional, and international regulations. He establishes and coordinates Technical Working Groups, conducts capacity building initiatives, develops and administers Biosafety and Biosecurity training programs, establishes legislative and technical frameworks, and ensures compliance. Previously, he served as an expert for the African Society for Laboratory Medicine (ASLM), lecturer, Quality Management System Consultant, and a Laboratory Mentor.
Yenew Kebede Tebeje
Yenew Kebede Tebeje is a medical microbiologist and public health expert with over 20 years of clinical, teaching, laboratory science, research, capacity building, and programme design and management experience. He currently serves as Head of Division of Laboratory Systems and Networks at Africa CDC where he leads programmes to strengthen public health laboratory systems and networks in Africa. Before joining Africa CDC, Dr Tebeje worked for more than 12 years as Technical Officer and later as Branch Chief for Laboratory at the US CDC in Ethiopia, where he provided strategic leadership for one of the most successful laboratory systems development programmes of the US CDC. He also worked as assistant lecturer, lecturer and later as assistant professor at Gondar College of Medical Science, University of Gondar. He was recognized several times for his outstanding contributions, including the Mission Honor Award, Meritorious Honor Award and US CDC Center for Global Health Director’s Award. He has written lecture notes on microbiology, immunology and parasitology and authored and co-authored more than 30 articles in peer-reviewed journals. He holds Doctor of Medicine Masters in Medical Microbiology and Master of Public Health degrees.
Donewell Bangure
Donewell Bangure is Medical Laboratory Scientist and Epidemiologist with African CDC with more than 15 years of work experience in public health laboratories across Africa. He was among the first set of field epidemiologist responders for the African Union Support for Ebola Outbreak in West Africa (ASEOWA) in 2014-2015 and one of the first epidemiologists to respond to the 9th and 10th Ebola Virus Disease outbreaks in Democratic Republic of Congo in 2018. Before Africa CDC, he served for two years as Field Coordinator for the Zimbabwe Field Epidemiology Training Programme and as a Principal Medical Laboratory Scientist for 10 years with the Ministry of Health and Child Care, Zimbabwe. He was part of the team of experts that conducted the Joint External Evaluation for Liberia and Afghanistan, which assessed their capacity under the International Health Regulations (2005). He played a key role in the response to the mudslide disaster that killed more than 500 people in Sierra Leone in August 2017. He is a graduate of the Africa CDC Fellowship programme with skills in event-based and indicator-based surveillance and management of public health emergency response. He has published more than 55 scientific papers in peer reviewed journals.
Ahmed E Ogwell Ouma
Ahmed E Ogwell OUMA currently serves as the Deputy Director General of Africa CDC where he works closely with African Union Member States and partners to deliver on the mandate of Africa CDC. He has over 25 years of experience in public health in different settings ranging from the national government, non-governmental organisations to the United Nations system and the African Union. He has expertise in health emergencies, preventing and controlling non-communicable diseases (NCDs), Influencing health policy and practicing global health diplomacy. He led the operations of Africa CDC during the COVID-19 pandemic, coordinating the planning, acquisition, and delivery of life-saving health products to African countries including test kits, personal protective materials, therapeutics, and vaccines. He previously worked with the Office for International Health Relations and the department for NCDs at the Ministry of Health of Kenya. Internationally, he is recognised for leading the negotiations for the Tobacco Control Treaty, contributing to the implementation of the WHO Framework Convention on Tobacco Control (WHO FCTC) particularly in the African continent, pioneered the intergovernmental working group on Public Health, Innovation, and Intellectual Property in Africa. He holds Bachelor of Dental Surgery, Masters in Public Health and Master of Philosophy in Human Behaviour.
References
- Africa CDC. (2019). Africa CDC biosafety and biosecurity initiative. Retrieved July 17, 2022, from https://africacdc.org/programme/laboratory-systems-and-networks/biosafety-and-biosecurity/
- Africa CDC. (2021a). Africa CDC biosafety and biosecurity initiative 2021 - 2025 strategic plan – Africa CDC. Retrieved July 17, 2022, from https://africacdc.org/download/biosafety-and-biosecurity-initiative-2021-2025-strategic-plan/
- Africa CDC. (2021b). Africa CDC biosafety and biosecurity initiative report on the consultative process to identify priorities for strengthening biosafety and biosecurity – Africa CDC. Retrieved July 17, 2022, from https://africacdc.org/download/africa-cdc-biosafety-and-biosecurity-initiative-report-on-the-consultative-process-to-identify-priorities-for-strengthening-biosafety-and-biosecurity/
- Africa CDC. (2022a). Development of a national biosafety and biosecurity strategy. https://africacdc.org/download/development-of-a-national-biosafety-and-biosecurity-strategy/. [Retrieved August 19, 2022].
- Africa CDC. (2022b). The regional training and certification program for biosafety and biosecurity professionals. Retrieved August 30, 2022, from https://africacdc.org/download/the-regional-training-and-certification-program-for-biosafety-and-biosecurity-professionals/
- Africa CDC. (2022c). The regulatory and certification framework for institutions handling high risk pathogens in the Africa region. Retrieved August 30, 2022, from https://africacdc.org/download/the-regulatory-and-certification-framework-for-institutions-handling-high-risk-pathogens-in-the-africa-region/.
- Global Health Security Index. (2019). Global Health Security Index Report. Retrieved July 12, 2022, from https://www.ghsindex.org/wp-content/uploads/2019/10/2019-Global-Health-Security-Index.pdf
- Gordon, P. (1961). The cybernetics of evolutionary process and of self-organising systems. 3rd Intern conf on cybernetics. Gauthier-Villars.
- World Health Organization. (2019). World Health Organization joint external evaluations. Retrieved July 12, 2022 from https://www.who.int/emergencies/operations/international-health-regulations-monitoring-evaluation-framework/joint-external-evaluations#:~:text=A%20Joint%20External%20Evaluation%20(JEE,to%20deliberate%20or%20accidental%20events.
- World Health Organization. (2022). National Action Plans for Health Security. Retrieved July 16, 2022, from https://extranet.who.int/sph/naphs