Abstract
CNS infections are associated with significant morbidity and mortality. The epidemiology of CNS infections shows striking differences in geographic regions. We reviewed the literature on clinico-epidemiological features of community-acquired CNS infections in Iran. Our review highlighted that the causes of CNS infections in Iran are diverse but information regarding the epidemiology and precise estimates of the burden of disease are lacking for most neuroinfections. Enteroviruses, S. pneumoniae, and Klebsiella species are the most commonly reported causes of viral, bacterial, and neonatal meningitis, respectively, whereas neurotuberculosis and neurobrucellosis place a huge burden. Improving the national surveillance system and implementing a nationwide data registry system for CNS infections are necessary to provide practically useful information regarding the microbial spectrum and the burden of CNS infections to suggest optimal preventive, diagnostic, and therapeutic strategies.
CNS infections continue to be associated with high rates of morbidity and mortality [Citation1,Citation2]. Despite the advent of therapeutic and prophylactic measures, the overall burden of CNS infections remains high [Citation1,Citation3]. The burden of CNS infections is not limited to early morbidity and mortality. They are associated with a high rate of long-term neurological sequelae and increased risk of later development of psychotic illnesses [Citation4], neurodegenerative disorders, and potentially dementia [Citation5,Citation6].
The burden of CNS infections is unequally distributed in different geographic regions and predominantly impacts low- and middle-income countries [Citation7]. A recent meta-analysis estimated that the incidence of CNS infections in low- and middle-income countries is 726 and 299 cases per 100,000 population, respectively, whereas the rate for high-income countries is 11 cases per 100,000 population [Citation8].
The nervous system may be infected by a diverse spectrum of microorganisms [Citation9]. Globally, infections that cause significant nervous system morbidity include viral (e.g., HIV, rabies, Japanese encephalitis virus, herpesviridae, dengue virus and chikungunya virus), bacterial (e.g., tuberculosis, syphilis, and bacterial meningitis), fungal (e.g., cryptococcal meningitis) and parasitic (e.g., malaria, neurocysticercosis, neuroschistosomiasis and soil-transmitted helminths) infections [Citation10]. However, the epidemiology of CNS infections shows striking differences in geographic distribution. The prevalence differs not only by geography and the capacity of vectors to transmit efficiently, as observed for arthropod-borne viruses but also along with the socioeconomic status of the community [Citation11] and immune status and comorbidities of the population [Citation11].
Iran is a large country that is located in the World Health Organization (WHO) Eastern Mediterranean Region (EMR), with a total area of 1,648,195 km2 and more than 80,000,000 population. It borders the Caspian Sea, Persian Gulf, and the Gulf of Oman, and also shares its northern borders with Armenia, Azerbaijan, and Turkmenistan, western borders with Turkey in the north and Iraq in the south, and eastern borders with Afghanistan in the north and Pakistan on the south. Diverse climatic conditions from hyper-arid to humid across the whole country [Citation12–14] provide a range of ecological conditions within which a wide spectrum of infective agents and their vector organisms can survive and transmit different infectious disorders. In spite of dozens of published papers that have addressed different types of CNS infections in Iranian patients, information regarding the epidemiology and precise estimates of the burden of disease are lacking for most neuroinfections in Iran. Therefore, in the present study, we systematically reviewed the available literature on clinico-epidemiological CNS infections in Iran and described the spectrum of causative pathogens based on their clinical syndromes.
Search strategy
PubMed was systematically searched for publications up to April 2020 using the following keywords: Iran, “Central Nervous System Infections” [Mesh], “Meningitis” [Mesh], “Encephalitis” [Mesh]), “Meningoencephalitis” [Mesh]), “Brain Abscess” [Mesh]), “Epidural Abscess” [Mesh]), “Empyema, Subdural” [Mesh], “neurolisteriosis”, “Tuberculosis, Central Nervous System” [Mesh], “neurotuberculosis”, “Paraparesis, Tropical Spastic” [Mesh]), “HTLV-I Infections” [Mesh], “Myelitis” [Mesh], “Echinococcosis” [Mesh], “AIDS Dementia Complex” [Mesh], “Neurosyphilis” [Mesh], “Poliomyelitis” [Mesh], “Rabies” [Mesh], “Malaria, Cerebral” [Mesh], “Neuroaspergillosis” [MeSH], “cerebral aspergillosis”, “cerebral Mucormycosis”, “Neurocysticercosis” [Mesh], “Rabies” [Mesh], “Meningitis, Cryptococcal” [Mesh], “Brucellosis” [Mesh], “neurobrucellosis”, “Lyme Neuroborreliosis” [Mesh], “neuroborreliosis”, “Chikungunya virus” [Mesh], “Dengue” [Mesh], “Zika Virus” [Mesh].
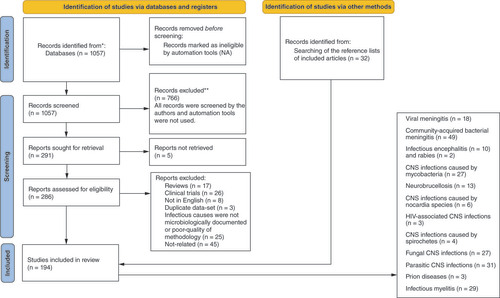
We included observational studies that described the demographic, clinical, and laboratory features of microbiologically-proven community-acquired CNS infections in Iranian patients including cohorts, case series and reports, and autopsy studies. Additional articles were identified through a review of reference lists, PubMed’s related articles feature, and the literature that cited the included articles to prevent missing any relevant data ().
All cases were classified into the following categories and discussed based on a syndrome-based approach: acute viral encephalitis, acute viral meningitis, acute bacterial meningitis, chronic meningitis, brain abscess, infectious myelitis, CNS infections caused by intracellular bacteria, CNS infections caused by spirochetes, fungal CNS infections, parasitic CNS infections, prion diseases, and HIV-associated CNS involvement.
Review
Acute viral encephalitis
Encephalitis is an important cause of morbidity, mortality, and permanent neurological disability in both adults and children. In 2019, the global burden of encephalitis has been estimated as high as 1,444,720 incident cases, 89,900 deaths, and 4.80 million disability-adjusted life years (DALYs) [Citation15]. Encephalitis can be due to a wide variety of infectious and autoimmune causes [Citation16] and 20–50% of encephalitis cases with a known cause are attributed to viruses [Citation17]. The most common cause of viral encephalitis is HSV encephalitis, with a high fatality rate of up to 70% if untreated [Citation17,Citation18]. Our literature review also identified HSV as the most common cause of encephalitis in about 86% of episodes of microbiologically documented viral encephalitis reported in Iran, with a mortality rate of 4–46.6% (Supplementary Table 1) [Citation19–21]. Although much less frequent, VZV was one of the most frequently identified causes of encephalitis in Iranian patients [Citation21,Citation22]. Except for rare cases of EBV encephalitis [Citation23], our review found no report of encephalitis caused by other members of the herpesviridae family in the literature.
Other notable causes of viral encephalitis include arthropod-borne encephalitis viruses (arboviruses) which account for a significant global public health problem [Citation24]. Worldwide, the most prevalent arboviral diseases are dengue fever, chikungunya fever, Zika virus (ZIKV) infection, yellow fever, Japanese encephalitis (JE), West Nile virus (WNV) infection [Citation25], and tick-borne encephalitis (TBE) [Citation26].
WNV is a widely distributed arbovirus with extensive distribution throughout Africa, the Middle East, parts of Europe, the former Soviet Union, south Asia, Australia and North America [Citation27]. In some parts of the world, WNV is the second most common cause of viral encephalitis after HSV [Citation28]. WNV-specific antibodies were detected in the general population of several countries in the EMR such as Iraq, Egypt, Jordan, Lebanon, Libya, Morocco, Pakistan, Sudan, and Iran. Symptomatic infections are also reported in Pakistan, Tunisia and Iran [Citation29]. WNV infection is relatively common in Iran [Citation30] and the virus has been isolated from mosquitoes, horses, and humans [Citation31–33]. According to the older literature, the highest seroprevalence of WNV in humans was identified in central and southwestern Iran, including the Khuzestan province, while it was low in the north of the country [Citation34]. More recent studies, however, reported an overall seroprevalence of 1.5–20.6% for WNV [Citation31,Citation35–38]. Between 2008 and 2009, the virus was detected in both CSF and serum of 1.2% of patients with encephalitis in Isfahan, Iran [Citation39]. Thus, it should be considered among the potential etiologic agents of acute encephalitis and included in the routine virologic testing for encephalitis in Iran.
Tick-borne encephalitis virus (TBEV) is also an important cause of encephalitis in Eastern, Central, and Northern European countries, Northern China, Mongolia, and the Russian Federation [Citation40], accounting for about 3.5% of all the community-acquired CNS infections [Citation41]. In Europe and Asia between 10,000 TBE cases are reported annually but this rate is believed to be significantly lower than the actual total number of clinical cases [Citation42]. Our literature review found no evidence of the circulation of TBEV in the EMR. Of the two main vectors of TBEV, Ixodes ricinus is found in large parts of Europe, extending as far as Turkey, Northern Iran, and the Caucasus in the Southeast [Citation43]. Despite the distribution of TBEV vectors at least in Northern provinces, we found no report of human infection with the TBE in Iran [Citation44]. Given that this virus is not routinely tested in cases with suspected CNS infections, it might be underdiagnosed in patients with acute encephalitis in the country. With the increase of tourism, TBE has become a more global problem and should be included in the differential diagnosis of CNS infections not only for those living within an endemic region but in case of an appropriate epidemiological clue- even those living outside endemic areas. It has been estimated that an overall risk of acquiring TBE for a non-vaccinated tourist staying in a highly endemic region for four weeks during the TBEV transmission season is approximately 1 case per 10,000 person-months of exposure which is approximately equivalent to the risk of contracting typhoid fever or malaria while traveling in India [Citation45].
JE, the most commonly identified epidemic cause of viral encephalitis, has been estimated to occur in approximately 70,000 cases each year, mostly in Asia and northern Australia [Citation16]. It is estimated to be responsible for the loss of 709,000 DALYs annually [Citation46]. Despite its widespread distribution and extensive vaccination efforts for the prevention of JE in Asia [Citation47], the role of JE in Iran is unclear. According to the WHO, no case of JE has been reported in EMR between 2006 and 2021 [Citation48]. Whether this is an underdiagnosing, underreporting, or it does not exist in Iran and its neighbors needs to be investigated in future research.
Dengue virus is another arbovirus [Citation49] that can involve the entire neuroaxis and presents with a variety of neurological complications including encephalitis [Citation50]. Globally, dengue fever is the most rapidly spreading mosquito-borne viral infection with ongoing geographic expansion to new countries. More than 70% of the population at risk for dengue worldwide live in member states of the WHO South-East Asia Region and Western Pacific Region [Citation51]. The WHO estimates that 50 to 100 million infections occur annually with an estimated 500,000 cases of severe disease and about a 2.5% death rate [Citation52]. Recent dengue fever outbreaks in the WHO EMR in the last five years (2016–2020) occurred in Sudan, Yemen, Oman, and Pakistan. Several factors have been proposed as contributing to the emergence and rapid transmission of dengue in the region, including the rapid prevalence of the main dengue vectors, vulnerability, favorable environmental conditions, and unplanned expanding urbanization [Citation53]. In Iran, dengue fever has started to be a concern since 2008, after the first case with a travel history to Malaysia was confirmed. Subsequently, evidence of the circulation of the dengue virus has been documented by a retrospective study (2000–2012) which found 5% and 1% of 300 specimens were positive by serology and serology plus PCR for dengue virus infection, respectively. In total, 48% of these seropositive cases did not have any travel history outside Iran [Citation54]. Until now, however, no case of neurological involvement by this pathogen has been reported in Iran.
Chikungunya (CHIKV), another emerging cause of encephalitis, is an Old-World alphavirus with a worldwide distribution. Available evidence suggests increasing neurovirulence of the virus, particularly among critically ill patients [Citation55]. Following the introduction of the CHIKV in the mid-1950s, numerous small outbreaks occurred in Africa. However, massive outbreaks were noted in Thailand in the late 1950s and early 1960s, and in India from the early 1960s into the 1970s [Citation56]. The global expansion of the virus has occurred over the last decade [Citation55]. The first outbreak of chikungunya fever in the EMR occurred in 2010–2011 in Al-Hudaydah, Yemen, and affected about 15,000 people [Citation57]. More recently, the largest outbreak of chikungunya fever in Africa and EMR occurred in Sudan, with a total of 48,763 cases between 31 May 2018 and 30 March 2019 [Citation58]. Though CHIKV infection is not endemic in Iran, there are a few reports that show evidence of the circulation of CHIKV in the country. Bakhshi et al. detected CHIKV of the Asian genotype in mosquito pools collected in the North Khorasan and Mazandaran provinces [Citation59]. A seroprevalence study by the Department of Arboviruses and Viral Hemorrhagic Fevers of Pasteur Institute of Iran also showed evidence of the introduction of CHIKV to Iran, which were closely related to the strains in Pakistan [Citation60]. However, we found no report of symptomatic CHIKV infection or its neurological involvement published in Iran. Considering the evidence of the circulation of the virus in the country, CHIKV should be considered among the potential causative pathogens of encephalitis in Iran, particularly in critically ill patients.
Zika virus (ZIKV) is another emerging arbovirus [Citation61] that has been responsible for several outbreaks of ZIKV disease in Africa, America, Asia, and the Pacific [Citation62]. Congenital and non-congenital infections of the CNS by ZIKV have been described [Citation63]. In spite of the worldwide distribution of ZIKV, it is not known as an endemic or emerging neuropathogen in Iran. A recent study investigated the presence of ZIKV IgG-specific antibodies and the genome of ZIKV in human serum samples, as well as in wild-caught mosquitoes in urban and rural areas of the Hormozgan province, Iran: no evidence of ZIKV infection in people or circulation of the virus in any of the vectors was identified [Citation64].
The epidemiology of encephalitis has changed over time. Encephalitis is a well-recognized complication of measles infection and sometimes can be its sole manifestation [Citation65]. One in 1000 cases of measles is estimated to develop encephalitis [Citation66]. In 1997, all EMR countries adopted a resolution to eliminate measles by 2010. Between 2013 and 2018, 144,966 cases of Measles were reported in the EMR [Citation67]. Iran received a certificate for measles elimination in October 2019 when the EMR was experiencing its greatest upsurge in measles cases [Citation68]. Before the inclusion of the measles vaccine in the Iranian national immunization program, 83% of children had been in contact with measles by the age of 12 years [Citation30]. As its prevalence has fallen markedly in recent decades, most information concerning neurological complications of measles including subacute sclerosing panencephalitis (SSPE) comes from the older literature. An old study described 200 cases of SSPE in the decade from 1975 to 1984 in Tehran, Iran [Citation69]. Recently, vaccine hesitancy and the resurgence of measles infection in some countries including Iran’s neighbors have raised concerns regarding an increase in patients with neurological complications of measles [Citation70,Citation71]. Notably, in 2019, nearly 870,000 cases of measles were reported worldwide, with almost 210,000 deaths, mostly in young children which was the highest level in decades [Citation71]. Cross-border travel due to the recent instability in Afghanistan which is endemic for measles has increased the risk of the spread of the virus to Iran. Preventing a resurgence of measles in the country needs controlling indigenous transmission through efficient vaccination coverage in at-risk subpopulations, improving disease surveillance, and rapid outbreak containment.
Rabies, a fatal zoonotic disease caused by Lyssaviruses [Citation72], has been distributed worldwide and can affect all mammals including humans. Of its two major clinical forms (encephalitic and paralytic), the encephalitic form is more common in humans [Citation73]. Despite the introduction of an effective vaccine, about 59,000 deaths occur annually due to rabies worldwide [Citation73]. In Iran, rabies has been reported in almost all 31 provinces of the country [Citation74] and dog-related rabies is responsible for most of the bite-related cases [Citation75]. Although the mortality rate of human rabies has decreased from 0.9/1,000,000 population in 1980 to 0.02–0.03/1,000,000 in recent years, it still kills 2–6 persons annually in the country [Citation74]. Despite the overall incidence remains significant, only a few studies have described patients with rabies, with a mortality rate of 100% [Citation75,Citation76]. One of these studies reported 463 suspected cases of rabies from different geographic regions in Iran of whom 14 cases had positive real-time PCR and fluorescent antibody test (FAT) for the virus [Citation75]. Iran faces many challenges in the elimination of rabies including the lack of rabies awareness, particularly in rural areas, inadequate rabies vaccination of dogs and cats, and easily spread of rabies over borders into Iran. These make control of rabies extremely difficult [Citation77].
The influenza virus is associated with a wide variety of neurological complications [Citation78]. The most worrisome complication arising from neurovirulent strains of influenza is rapidly progressive necrotizing encephalitis which can be fatal or cause permanent neurological sequelae [Citation79]. The incidence of influenza-related acute encephalitis is estimated at 1–2/100000 episodes of symptomatic infections [Citation16]. In 2009, out of 3672 confirmed cases of influenza A (H1N1) in Iran, meningitis and encephalitis were reported in 11% of 140 fatal cases of influenza infection [Citation80]. Although mild neurologic symptoms were common and reported in up to 42% of hospitalized patients with H1N1 infection, severe neurologic complications occurred in only 9% of inpatients in a single-center study [Citation81]. Thus, influenza should be considered in the differential diagnosis of patients with encephalitis, especially if they present with respiratory symptoms or during an influenza outbreak.
Viral meningitis
Viruses are the most common cause of aseptic meningitis syndrome [Citation9]. Viral meningitis is more common in children and the incidence decreases with age [Citation82] but it can occur at any age [Citation83]. Currently, in countries with high vaccine coverage rates, viral meningitis is more common than bacterial meningitis, with an estimated incidence of 0.26–17/100,000 population [Citation84]. The burden of viral meningitis remains uncertain but it poses a major public health challenge, especially in developing countries [Citation85]. Globally, enteroviruses are the most common causes of viral meningitis, followed by herpesviridae, parechoviruses, mumps virus, HIV, and arboviruses [Citation86,Citation87]. The review of the published literature in Iran also identified enteroviruses as the most common cause of viral meningitis in 70% of microbiologically proven cases of viral meningitis reported in the literature, followed by mumps virus, VZV, and parechoviruses (Supplementary Table 2). Concomitant viral and bacterial meningitis and viral meningitis caused by more than one viral pathogen have also been reported [Citation88–91].
Almost any of the enterovirus types can give rise to neurological manifestations [Citation82]. Following the elimination of poliovirus infections in the majority of countries, non-polio enteroviruses are emerging as the causative agents of CNS involvement [Citation92]. Currently, enterovirus 71 (EV71) is considered to be the most dangerous enterovirus [Citation93]. Outbreaks of EV71-associated CNS infections have recently been reported in Asia, and sporadic cases occur in USA and Europe [Citation92]. In Iran, 33 cases of EV71 meningitis have been reported until 2020 and this pathogen accounted for 21.3% of enteroviral meningitis in one study [Citation93,Citation94].
Over the last decades, mumps virus has been the second most frequently reported causative pathogen in Iranian patients with viral meningitis. Before the widespread immunization against mumps, it was a common cause of meningitis in an unimmunized population, which occurred in 15% of patients with mumps [Citation82]. With the introduction of the Measles-Mumps-Rubella (MMR) vaccine [Citation9], the incidence of mumps-associated meningitis has dramatically declined to <1% of all cases of meningitis and encephalitis in most countries [Citation95,Citation96]. MMR vaccine has been included in the Iranian national immunization program since 2003 [Citation97] and no report of mumps-related meningitis in Iran has been reported in the literature since 2013. Even though the MMR vaccination has significantly reduced the incidence of infections caused by viruses contained in the vaccine, it has been reported to be associated with a variety of neurological adverse reactions, including aseptic meningitis. Cases of aseptic meningitis following immunization with the MMR vaccine have been also described in Iran [Citation98,Citation99]. In a report of 65 children with aseptic meningitis in Tabriz, Iran (2004–2005), about 70% of them had a history of MMR vaccination within 30 days before hospital admission [Citation100].
Herpesviruses are among the major causes of viral meningitis worldwide [Citation101]. HSV (most commonly HSV type 2) now ranks second among viral pathogens of meningitis in adolescents and adults in developed countries [Citation82]. Of herpesviridae, only VZV was identified as a common cause of viral meningitis in Iran [Citation88,Citation102–104]. Despite the introduction of effective VZV vaccines in many developed countries, it is not part of the routine immunization schedule in Iran [Citation105] and by the age of 40 years and more, most individuals had already been infected by VZV [Citation106]. Considering that CNS is the most common non-cutaneous site of involvement after chickenpox [Citation107], VZV-associated neurological complications do not infrequently occur in infected individuals.
HSV, HHV-6, HHV-7, EBV, and CMV have been rarely reported as the causative pathogens of aseptic meningitis. Out of 1736 patients with suspected viral infection in Iran that reported in the literature, 419 microbiologically proven viral meningitis were described, of which VZV, HHV-6, and HHV-7 accounted for 10.5%, 1.7%, and 0.5%, respectively, and HSV and CMV were identified rarely [Citation88,Citation90,Citation102,Citation103,Citation108,Citation109]. All studies that reported patients with viral meningitis included patients in the pediatric age groups except two that studied a mixed population of children and adults. Furthermore, among non-case report articles, 11 studies only tested patients for one or two viral pathogens. So, a selection bias may influence the proportion of any given etiologic diagnosis.
The influenza virus, although reported as an important cause of meningitis and encephalitis during the 2009 H1N1 influenza virus pandemic, has not been identified as a major cause of aseptic meningitis in the more recent publications in Iran [Citation80]. Other viral pathogens including parainfluenza virus, lymphocytic choriomeningitis virus (LCMV), CHIKV, arboviruses (WNV, sandfly-associated viruses, ZIKV, and Dengue virus), and HIV were not identified as important causes of meningitis in Iranian patients. LCMV is an important cause of viral meningitis which utilizes rodents as its principal reservoir [Citation110]. Despite the occurrence of LCMV infection in Iran’s neighboring countries [Citation44], no case of LCMV infection or CNS involvement caused by this pathogen has been reported in Iran. Sandfly-associated viruses (Sicilian, Naples, and Toscana) which are endemic in the Mediterranean area, occasionally cause aseptic meningitis [Citation111]. Among them, the Toscana virus shows a strong neurotropism [Citation112]. Transmission of sandfly-associated arboviruses has been documented in Iran [Citation113,Citation114] and the Sicilian virus and Naples virus are the most commonly reported serotypes in the country [Citation115]. However, to date, no case of meningitis caused by these pathogens has been reported in Iran.
Acute bacterial meningitis
Community-acquired bacterial meningitis is a potentially fatal neuroinfection that is associated with a substantial burden of disease. By far the highest burden of meningitis is estimated to occur in low- and middle-income countries [Citation116]. Although over the past 10–20 years, the annual incidence of bacterial meningitis in Western countries gradually declined, the incidence is still substantially high in low-income countries [Citation117]. The highest burden of disease is seen in a region of sub-Saharan Africa, known as the African Meningitis Belt, especially recognized to be at high risk of epidemics of meningococcal but also pneumococcal meningitis. Meningitis is also a great concern in Iran: nationally in 2016, 58,229 DALYs were estimated to be associated with meningitis [Citation116]. It is a notifiable disease in Iran. The national notification system for meningitis was established in 1981. Using reporting data from the national notification system, the incidence rate of meningitis in Iran was estimated at 3/100,000 population in 1984, which decreased to 0.5/100,000 population in 1988. In 1996, the incidence rate showed an increase that could be related to the increase in the number of individuals acquiring meningitis or an improved surveillance system. In 2001, the number of meningitis cases showed an increasing trend to as high as 3.8/100,000 population and since then, it has declined steadily which could be due to the underreporting or actual decrease in the number of meningitis cases [Citation118]. A nationwide study of bacterial meningitis that was performed before the inclusion of pentavalent vaccination (i.e., hepatitis B, diphtheria, pertussis, tetanus, Haemophilus influenzae type b) in the national immunization program in 2014, reported the incidence of bacterial meningitis in Iran as high as 0.28/100,000 population in 2008 and 0.09/100,000 in 2014. However, the reported incidence rates are far lower than the reported incidence of bacterial meningitis in other high- [Citation117,Citation119], middle- and low-income countries [Citation120]. So, it seems to be a gross underestimation. Another report on the incidence of meningitis in Tehran, Iran (1999–2005) has estimated the incidence rate of 1/100,000 to 12.8/100,000 populations for meningitis in different age groups [Citation121]. However, it was an overall estimation of meningitis and did not report the incidence of bacterial versus viral meningitis. The epidemiology of bacterial meningitis has been dynamically changing over the past 30 years following the introduction of conjugate vaccines targeting H. influenzae type b, Streptococcus pneumoniae, and Neisseria meningitidis [Citation117]. According to the published literature, the most frequently reported pathogen as the cause of community-acquired bacterial meningitis in Iran was S. pneumoniae, followed by N. meningitidis, H. influenzae, and S. agalactiae (GBS) (Supplementary Table 3). The studies aimed to investigate the distribution of capsular types of invasive S. pneumoniae isolates (2000–2019) showed that serotypes 23F (16.4%) was the most circulatory serotype followed by 19F, 19A, 6A/B, 9V, and 11A [Citation122]. However, in a series of 114 patients with bacterial meningitis in a pediatric population in some hospitals in Tehran, Iran, serotype 18C was the most common serotype, followed by serotypes 14, 19A, 6A, 7F, 4, 3, 9V, 8, and 23F [Citation123]. In this study, N. meningitidis group B was the most frequent serotype isolated in 51% of those with meningococcal meningitis, followed by groups C, A, Z, and W135 [Citation123].
Of conjugate vaccines, only H. influenzae type b childhood vaccination is routinely performed in Iran. A more recent clinico-epidemiological study showed a 46.2% decrease in cases of H. influenzae type b meningitis at all ages following pentavalent vaccination in Iran (November 2014 to July 2018) as compared with the period before vaccination. Pentavalent vaccination was also associated with a decrease in the overall mortality rate of bacterial meningitis from 2.7% to 1.2% [Citation124]. Over the same period, the number of cases of meningitis caused by S. pneumonia also decreased but there was an increase in the incidence of meningococcal meningitis [Citation124]. Currently, no nationwide mass vaccination program against N. meningitidis is performed in Iran but high-risk groups including military trainees and pilgrims on their way to Mecca, Saudi Arabia are routinely vaccinated against N. meningitidis [Citation125]. The meningitis belt is highly connected by Muslim pilgrims taking part in the annual Hajj ceremony, gathering in Saudi Arabia, as the congestion of people promotes increased carrier rates of meningitis [Citation67]. After the epidemics caused by N. meningitidis serogroup W135 among Hajj pilgrims in 2000 and 2001, the Ministry of Health of Saudi Arabia required all pilgrims to be vaccinated with the tetravalent (A, C, Y, W135) polysaccharide vaccine [Citation123]. Military personnel and recruits are another high-risk group for meningococcal disease, with a reported incidence of four to ten-times greater than that of the general population [Citation126]. In an investigation between 2000–2004 in Iran, the incidence of meningococcal meningitis among military personnel showed a sharp decline following vaccination at least two weeks before arriving at military training camps; however, sporadic cases of the disease did occur [Citation125].
The highest incidence of bacterial meningitis in all age groups has been reported in infants younger than three months. Neonatal meningitis causes mortality in 5–20% of cases, and 20–50% of cases develop neurological sequelae, even in high-income countries. While studies in developed countries have generally found Streptococcus agalactiae (GBS), Escherichia coli, and Listeria monocytogenes, as well as Klebsiella pneumoniae and Enterobacter species in preterm infants, as the main causes of neonatal meningitis [Citation127], a systematic review published in 2011 described how in the low- and middle-income world results have varied; particularly regarding GBS, other Gram-negatives, L. monocytogenes and Gram-positive organisms [Citation128]. Published literature from Iran that focused on meningitis in newborn infants reported Klebsiella species as the most common causative pathogen in 25% of reported neonatal meningitis. GBS as the second most common cause accounted for 12% of reported culture-positive neonatal meningitis [Citation129–131]. Other causative pathogens in decreasing order of frequency include enterococci, E. coli, H. influenzae, and Acinetobacter species while S. pneumoniae, S. aureus, Pseudomonas species, M. catarrhalis, other streptococci, and Candida species have been rarely reported [Citation129,Citation130,Citation132,Citation133]. Nevertheless, the small number of studies and neonates included makes it hard to draw a conclusion from the results. Consistent with our results, another recent meta-analysis on the pooled prevalence of leading causes of neonatal sepsis in developing countries highlighted that Klebsiella species is the leading cause of neonatal sepsis in low- and middle-income countries [Citation134]. The majority of studies included in these reviews were retrospective, with varying methods, often using different inclusion and exclusion criteria and sometimes using different diagnostic methods [Citation128]. Thus, future well-designed prospective investigations are necessary for the validation of these findings.
In a high percentage of patients with presumed bacterial meningitis described in the Iranian literature, the causative agent could not be isolated from CSF or blood. While CSF culture is considered the gold standard for the diagnosis of acute bacterial meningitis with a high yield in the absence of prior antibiotic treatment [Citation135], only 10% of patients in the study of Berangi et al. had positive CSF culture, and 55.4% had negative CSF and blood cultures [Citation136]. Several factors can influence the yield of culture-based diagnostic tests in bacterial meningitis including antibiotic treatment prior to LP and types of neuropathogens [Citation137]. Berangi et al. reported a high rate of pre-treatment with antibiotics: 40% of patients had received antibiotics before LP [Citation136]. Insufficient microbiological capacity can be another constraint for cause-specific diagnosis of meningitis. In a previous study with the aim of external quality assessment of 310 Iranian microbiology laboratories for identification of the most common causative pathogens of meningitis, only 83%, and 16% identified S. pneumoniae and H. influenzae on CSF samples, respectively [Citation138]. So, low-quality microbiological diagnostics, as well as the high frequency of pretreatment with antibiotics, are two important contributing factors to the low yield of culture-based methods in the diagnosis of bacterial meningitis.
Our review also identified that a substantial proportion of published studies on bacterial meningitis in Iran suffer from limitations in design, definitions, and reporting. The majority of them did not define episodes of meningitis based on where they were acquired (i.e., community-acquired vs healthcare-associated), and did not analyze them separately [Citation139,Citation140]. Similarly, none of the two recently published systematic reviews and meta-analyses regarding bacterial meningitis in Iran considered this important point in their inclusion and exclusion criteria and analyzed a mixed population of patients with community-acquired and healthcare-associated meningitis [Citation139,Citation140]. This is not only the case for most of the studies that tried to investigate the microbial spectrum of bacterial meningitis, but also for those reported resistance patterns of the causative pathogens. These shortcomings might be responsible for reporting bacterial species that are typically the causes of healthcare-associated infections such as coagulase-negative staphylococci among the common causes of bacterial meningitis in these studies [Citation139,Citation141] and also influence the reported resistance rates to antimicrobials among bacteria which often seem to be higher than they really are.
A few studies reported the resistance rates of S. pneumoniae to penicillin (38–77%) and third generation cephalosporin (10–12%) [Citation142–145] in Iranian patients with pneumococcal meningitis. Nevertheless, the single-center design of these studies and the small number of cases make it difficult to draw a valid conclusion about the resistance rate of S. pneumoniae and its trend over time. Several other studies investigated the resistance rate of invasive pneumococcal isolates. In a study by Bokaeian in Zahedan (2008–2010), intermediate and high-level penicillin resistance was identified in 37.3% and 45.4% of isolates, respectively [Citation146]. Another study in Tehran (2013–2016) reported that 21% of invasive pneumococcal isolates (60% of isolates from the CSF specimens) were penicillin-non-susceptible [Citation147]. Serotype 19A was significantly associated with resistance to penicillin [Citation147].
Chronic meningitis
Chronic meningitis is defined as a meningeal disease with CSF inflammation lasting for four weeks or more, with failure of clinical improvement or clinical worsening [Citation148]. Although chronic meningitis accounts for less than 10% of all cases of meningitis, it is associated with significant morbidity and mortality [Citation149]. Most of the body of literature about chronic meningitis consists of case reports and a small number of retrospective case series from single centers. The causes of chronic meningitis are diverse and vary between countries and regions. There are few studies describing the characteristics of patients with chronic meningitis in the literature [Citation150,Citation151]. Globally, tuberculous meningitis and cryptococcal meningitis are the two most common types of chronic meningitis [Citation152]. In a multinational study on the epidemiology of community-acquired CNS infections (2012–2014), among neuroinfections, neurosyphilis, Brucella meningitis, neuroborreliosis, and CNS tuberculosis were significantly more likely to present chronic courses. Cryptococcal meningitis was the most common chronic CNS infection in individuals with HIV infection [Citation153]. A series of 97 (1989–1999) patients with chronic meningitis in Tehran, Iran reported tuberculous and Brucella meningitis as the most common infectious causes of chronic meningitis [Citation154,Citation155]. The majority of other literature on chronic meningitis in Iran described one or a small number of patients, or patients with a specific cause of chronic meningitis. Future well-designed clinico-epidemiological studies should focus on the burden of chronic meningitis and its etiologic agents, particularly in high-risk populations in Iran.
Brain abscess
Brain abscess is a focal, intracerebral infection consisting of a collection of pus surrounded by a well-vascularized capsule [Citation156]. The reported incidence ranges from 0.4 to 0.9 cases per 100,000 population [Citation157]. Despite a relatively low incidence rate, brain abscess remains a challenging clinical problem with substantial case fatality rates. During the past decade, the mortality of brain abscess has declined from 40% in 1960 to 10%–20% but many survivors continue to suffer from neurological deficits [Citation158]. The epidemiology of brain abscesses has changed with the increasing incidence of this infection in immunocompromised patients, particularly HIV-infected individuals, and solid organ and bone marrow transplant recipients, and the decreasing incidence of brain abscess related to sinusitis and otitis [Citation159]. Generally speaking, brain abscess is most frequently caused by oral cavity bacteria [Citation157]. However, it can be caused by other bacteria, mycobacteria, fungi, or parasites [Citation160].
The only investigation on brain abscesses in Iran was conducted by Faraji-Rad and Samini (1994–2004) in neurosurgical centers in Mashhad, describing 83 adults with brain abscesses. In this series, Streptococcus viridans was the most commonly isolated organism, followed by other streptococci, pseudomonas species, and enterococci. The mortality rate was 5%, and 24% of survivors experienced neurological sequelae [Citation161]. Other literature is mainly case reports or small case series. Future epidemiological studies should focus on the burden of brain abscesses in the general population and the high-risk groups as well as the current predisposing factors for the development of brain abscesses in Iran.
Infectious myelitis
Myelitis can involve the gray matter (anterior horn cell myelitis), or the white matter (transverse/longitudinal myelitis) of the spinal cord. Most cases of gray matter myelitis or acute flaccid paralysis are caused by the enteroviruses (polioviruses, enteroviruses 70 and 71, echoviruses, coxsackieviruses A and B) and the flaviviruses (WNV, Japanese encephalitis virus, tick-borne encephalitis virus). White matter transverse/longitudinal myelitis, on the other hand, is usually caused by the herpesviridae family (HSV, VZV, CMV, EBV) and influenza virus [Citation162].
Approximately 1% of wild poliovirus infections are associated with paralytic illness [Citation92]. With successful vaccine eradication campaigns, polioviruses are no longer recovered from patients with infectious myelitis in developed countries, and only rarely in developing countries, including Iran’s neighbors (Afghanistan and Pakistan) where poliomyelitis still occurs [Citation92,Citation163]. Iran is a wild poliovirus-free country since 2001 [Citation164]. All cases of poliomyelitis in Iran were published before 2018 however cases of vaccine-associated paralytic poliomyelitis have been rarely reported [Citation165] (Supplementary Table 4). The most frequently reported wild-type polioviruses in Iran were types 1, 2, and 3 [Citation166,Citation167]. Overall, cases of acute flaccid paralysis (AFP) were much less frequently reported in the post-polio eradication era. However, since 2012, clusters of AFP have been reported in Europe, Asia, South America, and the United States. Non-polio enteroviruses, particularly enteroviruses D68 (EV-D68) and more recently EV-A71 have emerged as potential causes in many of the cases identified [Citation168]. In Iran, a few studies investigated the causative pathogens of AFP. Between 2000 and 2002, of 288 cases of residual paralysis, 3 were caused by imported wild polioviruses, 17 were caused by vaccine-like polioviruses, and 239 were caused by different serotypes of non-polio enteroviruses (echoviruses 3–7, 9, 12, 18, 33, and coxsackie B5) [Citation169]. Another study with the aim of molecular typing of non-polio enteroviruses isolated from cases with AFP in Iran between 2010 and 2015 identified 9 different isolates of echoviruses, with echovirus 3 being the most commonly isolated serotype [Citation170]. In a more recent study in Iran, 20 types of non-polio enteroviruses were isolated from stool specimens of AFP cases collected by routine AFP surveillance activities during 2015–2018. These include echovirus 3 as the most common isolated serotype followed by echovirus 6, 7, 13, and 21, and coxsackie B viruses. Echovirus 3 and echovirus 6 strains detected in this study were closely related to Indian and Malaysian strains, respectively [Citation171]. Accordingly, non-polio enteroviruses should be considered among the main causes of AFP in Iran.
Other important causes of infectious myelopathy include HIV, T. pallidum, M. Tuberculosis, Brucella species, and human T lymphotropic virus type I (HTLV-1). HTLV-1 is estimated to infect at least 5–10 million people worldwide; however, its actual prevalence is unknown because of the lack of data in most parts of the world [Citation172]. The main highly endemic areas are the Southwestern part of Japan, some parts of the Caribbean area, and its surrounding regions, foci in South America, some areas of intertropical Africa and the Middle East, and rarely isolated clusters in Australo-Melanesia [Citation173]. Iran has the second rank for HTLV-1 prevalence after Japan. The first clinical report of HTLV-1 in Iran was from Mashhad, in the Northeastern Iran, in 1986 [Citation174]. Mashhad is one of the highly endemic areas for HTLV-1, with an overall prevalence of 2.1% [Citation175]. Blood transfusion, breastfeeding, and sexual transmission are the main risk factors for HTLV-1 transmission in Iran [Citation174]. HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a rare inflammatory disease causing unremitting and progressive neurological disorders [Citation172]. The lifetime risk of HAM/TSP has been estimated as 4 in 100 HTLV-1-infected people [Citation176,Citation177]. HAM/TSP is a major health problem in some parts of Iran, particularly Mashhad. Several studies have been published that described the characteristics of patients with HAM/TSP in Iran. Most of the cases were reported from Mashhad. However, cases were also reported in other provinces such as Tehran, Yazd, and Kurdistan (Supplementary Table 5). There are also reports of HAM/TSP in Iranian patients who immigrated to other countries [Citation165–170]. The educational program, as well as, highly sensitive screening tests seem necessary to reduce the transmission of the virus in the community [Citation174].
CNS infections caused by other intracellular bacterial pathogens
The intracellular microbes causing CNS disease are a diverse group of human pathogens and include L. monocytogenes, Salmonella, and Brucella spp., M. tuberculosis, C. burnetii, R. rickettsii, R. prowazekii, E. chaffeensis, and Tropheryma whipplei. They use a wide variety of mechanisms to enter their human hosts through contaminated food, the bite of infected arthropods, and inhalation [Citation178]. Among these intracellular neuropathogens, M. tuberculosis and Brucella spp. are major causes of neurological infectious diseases in Iran but others (L. monocytogenes, Nocardia species, Salmonella species, Bacillus anthracis, and C. burnetii) have been only rarely reported [Citation179–183].
Mycobacterial CNS infections
Infection of the CNS by Mycobacterium tuberculosis is the most feared and dangerous form of tuberculosis [Citation184], accounting for approximately 5 to 10% of all extrapulmonary TB cases and approximately 1% of all TB cases [Citation185]. Neurotuberculosis which is responsible for 5.8% of all community-acquired CNS infections worldwide [Citation41] is associated with a significant global burden of illness [Citation1]. Despite a decreasing trend in the overall incidence of TB in Iran, available data suggest that the Iranian population is greatly affected by tuberculosis, with an incidence rate of 10.9/100,000 (95% CI: 10.65–11.11) in 2018 [Citation186]. Considering the endemicity of TB in Iran and its high rate of morbidity and mortality, neurotuberculosis is estimated to be associated with a substantial burden of illness. However, given data limitations in this regard, future prospective cohorts of patients with neurotuberculosis should be conducted to estimate the mortality, morbidity, and overall burden of neurotuberculosis in Iran. Neshati et al. reported CNS TB in 8.1% of all newly diagnosed TB patients who were admitted to three referral hospitals in Mashhad, Iran [Citation187]. It accounted for 10% of 293 episodes of community-acquired febrile encephalopathy initially presumed to be CNS infections in adults hospitalized in a referral hospital in Mashhad, Iran [Citation22]. The most common clinical category of neurotuberculosis worldwide [Citation188] and in Iranian patients is TB meningitis, followed by intracranial tuberculoma and spinal TB arachnoiditis, respectively. Our literature review identified cases of neurotuberculosis mostly described in adults and predominantly in females. Two studies described neurotuberculosis in the pediatric-age groups [Citation189,Citation190] both were published before 2005. BCG vaccination coverage which is part of the Iranian neonatal vaccination program [Citation191] and has a documented protective effect against meningitis and disseminated TB in children [Citation192] has reduced the risk of tuberculosis and development of meningeal and miliary tuberculosis in children, particularly newborns. The reported mortality rate of neurotuberculosis ranged from 8–24% (Supplementary Table 6). Although older studies reported a higher rate of mortality as high as 42% [Citation189]. Data on functional and neuropsychological evaluation of survivors are lacking. Due to the endemicity and serious nature of this neuroinfection, providing accurate and detailed information regarding the disease burden in Iran is of utmost importance.
Although rare, meningitis due to non-tuberculous mycobacteria (NTM) has been also reported in Iran [Citation193,Citation194]. Of two reported cases in Iran, one occurred in a 21-year-old woman without underlying immunosuppression who presented with chronic meningitis caused by Mycobacterium chelonae [Citation193]. The second patient was a 6-month-old boy with a pineal region tuberculoma caused by Mycobacterium bovis as a complication of the Bacille Calmette-Guerin (BCG) vaccine [Citation194]. Thus, these microorganisms should be considered rare but potential causative pathogens of CNS infections in patients with compatible clinical syndromes and underlying predisposing conditions.
Neurobrucellosis
Although the incidence of human brucellosis has been reduced to a low level in Western European countries and the United States and eradicated in some other countries [Citation195,Citation196], it is among the most widespread zoonotic diseases, being endemic in the Middle East, Mediterranean Europe, Africa and many South American countries [Citation197]. Human brucellosis remains a huge burden in Iran [Citation198,Citation199], with an increasing trend in incidence. The number of patients has increased from 88,450 in 2009 to 198,030 in 2015, and mortality from 244 in 2009 to 578 in 2015 [Citation200]. Overall, neurobrucellosis is an uncommon complication that occurs in 3–10% of patients with brucellosis [Citation201–203] however it is frequently reported in countries that are endemic for brucellosis. The mortality rate is low but survivors are at significant risk of long-term neurological sequelae [Citation203]. Neurobrucellosis can be classified into three categories including acute meningitis or meningoencephalitis, chronic peripheral form (radiculopathy), and chronic CNS infection [Citation204]. Among patients who are admitted due to a variety of complications associated with brucellosis in Iran, 0.2–8% have brucella meningoencephalitis [Citation205–209]. There are a few case series that described the characteristics of patients with neurobrucellosis in Iranian patients, mostly reported from Tehran, Hamedan, and Shiraz [Citation206,Citation207,Citation210,Citation211] (Supplementary Table 7). The spectrum of clinical syndromes described in case series and case reports from Iran include acute, subacute, or chronic meningitis or meningoencephalitis, brain or epidural abscesses, myelopathy, polyradiculopathy, multiple cranial nerve palsies, cerebrovascular complications, and cerebral venous thrombosis. Patients with a variety of rare or atypical clinical manifestations were also described including personality or mood disorders and psychosis [Citation206,Citation207,Citation210–217]. In Iran, human brucellosis is predominantly associated with Brucella melitensis, with a much lower burden of B. abortus [Citation218]. Only one case series provided information regarding culture-confirmed cases of neurobrucellosis that reported positive blood or CSF culture in 5 of 31 episodes, all of them were caused by B. melitensis [Citation211].
CNS infections caused by Listeria monocytogenes
L. monocytogenes is one of the three most common causative pathogens of community-acquired bacterial meningitis in adults. CNS infection caused by L. monocytogenes can also present with neurological syndromes other than meningitis such as meningoencephalitis, cerebritis, brain abscess, and rhombencephalitis [Citation219]. In Iran, although available literature highlighted the presence of Listeria species in a variety of raw and ready-to-eat food samples [Citation220], information regarding the overall burden of human listeriosis or its neurological involvement is scares [Citation220,Citation221]. Whether a few reports of human listeriosis and CNS listeriosis are related to underreporting or underdiagnosis of listeria infections or the infrequency of CNS infections caused by this pathogen in the Iranian population needs to be investigated in future studies.
CNS infections caused by Nocardia species
Nocardiosis is caused by aerobic actinomycetes in the genus Nocardia and often occurs in immunocompromised hosts. CNS is among the most common sites of involvement and is affected in about 20% of cases. Species prevalence is not similar in different geographic regions [Citation222]. A systematic review and meta-analysis published in 2021 predominantly reported N. asteroides (21%), N. cyriacigeorgica (17%), and N. farcinica (10%) as the most common Nocardia species in Iran [Citation223]. Patients with cerebral nocardiosis have been described infrequently, mostly in immunocompromised patients. The most commonly reported neurological syndrome caused by nocardiosis in Iranian patients is multiple brain abscesses and the isolated Nocardia species are N. Cyriacigeorgica and N. Facanica. The underlying conditions of the reported cases of cerebral nocardiosis included diabetes mellitus, kidney transplantation, bone marrow transplantation, high-grade astrocytoma, and Behcet’s disease (Supplementary Table 8).
CNS infections caused by spirochetes
The most common spirochetes that cause CNS infections are Treponema pallidum, Borrelia species, and Leptospira species.
Neurosyphilis refers to the infection of the CNS by Treponema pallidum [Citation224]. Although it was common in the pre-antibiotic era, occurring in 25–35% of patients with syphilis, neurosyphilis is less common these days and most frequently seen in HIV-infected individuals [Citation225]. The overall prevalence of syphilis is relatively low in Iran, with reported prevalence between 0.2% to 0.6% [Citation226]. The prevalence of syphilis has been estimated at around 10/100,000 blood donors in Tehran province between 2005 to 2011 but it is more prevalent among high-risk groups (up to 7.2%), including HIV-infected individuals, sex workers, and prisoners [Citation227,Citation228]. Available data regarding neurosyphilis in Iran, however, is limited; we found only two case reports of congenital neurosyphilis and no case of neurosyphilis in adults in the literature [Citation229].
The genus Borrelia consists of two groups: species that cause Lyme disease and species that cause relapsing fever [Citation204]. Neurological involvement in tick-borne relapsing fever (TBRF) and Lyme disease is common, while it is rare in loose-borne relapsing fever (LBRF) [Citation230]. In Eurasia, TBRF was first described by Dschunkowsky [Citation1] in Ardabil, Iran, and it was called Borrelia persica (per’si.ca. L. fem. adj. persica, Persian which means Iran) [Citation231,Citation232]. The frequency of neurological manifestations of relapsing fever varies by Borrelia species, from none to >50%. The tick-borne species that are commonly associated with neurological complications are B. duttonii and B. turicatae and the louse-borne B. recurrentis [Citation230]. Available data suggest that TBRF is endemic in Iran: a prevalence of as high as 4 per million population has been reported in 2002. Ardabil province is the most infected area, followed by Hamedan, Zanjan, Kurdistan, and Qazvin provinces, respectively [Citation233]. Although hundreds of cases of borreliosis were confirmed in 19 provinces of Iran [Citation233], we only found one case report of neuroborreliosis, describing a 16-year-old boy with meningitis caused by Borrelia in Ardabil province [Citation234].
Globally, Lyme borreliosis is the most prevalent tick-borne zoonosis [Citation235] and neurological manifestations (often referred to as Lyme neuroborreliosis) are reported in up to 12% of patients with Lyme disease [Citation236]. Despite its high prevalence in Europe, North America, and Far Eastern countries [Citation235], Lyme borreliosis is not endemic in Iran. Our review identified only three case reports of Lyme borreliosis in the Mazandaran and Tehran provinces [Citation237–239] but no case of Lyme neuroborreliosis.
Leptospirosis comprises a group of zoonotic diseases that are endemic in many tropical regions and can cause large outbreaks following heavy rainfalls and flooding [Citation240]. Aseptic meningitis, with or without symptoms, is common in leptospirosis, occurring in up to 80% of cases [Citation9]. It is attributed to a host immune response to the Leptospires rather than to direct infection [Citation241]. In Iran, the seroprevalence of leptospirosis is high. The highest prevalence has been reported in the provinces located in the Caspian Sea coastal area [Citation242]. According to a systematic review in 2019, the seroprevalence of leptospirosis in humans is 27.8% (95% CI: 13.2–22.4) and 19.7% (95% CI: 6.7–32.6%) based on enzyme-linked immunosorbent assay (ELISA) and microscopic agglutination test (MAT), respectively. Also, a significant seroprevalence of leptospirosis in animals has been identified in Iran [Citation243]. Nevertheless, only a few cases of leptospiral meningitis have been reported [Citation244], and information regarding its burden of illness is limited. In a series of 20 cases of aseptic meningitis in Tehran, Iran, three cases of leptospiral meningitis have been described [Citation244]. Another case of leptospirosis complicated by multiple hemorrhagic lesions in the temporal and parietal lobes has been reported in Mazandaran province, Iran [Citation245]. Nevertheless, the patient had severe thrombocytopenia on presentation and intracerebral hemorrhage could be caused by a low platelet count.
Fungal CNS infections
Over the past two decades, fungal infections of the CNS have become significantly more prevalent. Invasion of the CNS by fungi largely depends on the immune status of the host and the virulence of the fungal strain. Fungal CNS infections are potentially fatal and associated with substantial morbidity in immunocompromised hosts. Cryptococcus neoformans, Aspergillus spp., and Rhizopus spp. remain the main fungal neuropathogens [Citation246].
Globally, C. neoformans is the most common cause of fungal meningitis [Citation9]. In large parts of the world with high rates of HIV infection, it accounted for the most common cause of adult meningitis [Citation247]. The majority of patients with cryptococcosis are immunocompromised [Citation248]. However, up to a third of non-HIV patients with cryptococcosis may have no known underlying immunosuppression [Citation249]. C. neoformans has been isolated from pigeons’ and swallows’ excreta, Eucalyptus and Stone fruit trees, and soil in different areas of Iran, including Northern, North-Eastern, Central, and Southern provinces [Citation250]. A few cases of C. neoformans meningoencephalitis have been reported in both immunocompetent [Citation251–253] and immunocompromised Iranian patients [Citation254–261], including HIV-infected cases and those with sarcoidosis [Citation256,Citation257], systemic lupus erythematosus (SLE) [Citation258], rheumatoid arthritis [Citation259], renal transplantation [Citation260], acute myeloid leukemia/hematopoietic stem cell transplantation (AML/HSCT) [Citation261], renal failure [Citation260], diabetes mellitus and asthma [Citation260] (Supplementary Table 9). Two cases of mixed TB and cryptococcal meningoencephalitis [Citation252,Citation257], one mixed cryptococcal and neoplastic meningoencephalitis [Citation261], and one mixed pulmonary TB and disseminated cryptococcosis, involving the CNS [Citation253] were also described. Most patients have been reported in Mazandaran Province, Hormozgan Province, Shiraz, Tehran, and Mashhad.
CNS aspergillosis is another fatal fungal neuroinfection that often occurs in severely immunocompromised patients [Citation9]. It can occur either in the setting of disseminated infection or from local extension of paranasal sinus infection. Isolated cerebral aspergillosis can occur in immunocompetent individuals or in the setting of injection drug use [Citation9]. Several cases of Aspergillus brain mass/abscess have been reported in immunocompetent and immunosuppressed patients in Iran. Aspergillus fumigatus was the main species isolated and A. niger was responsible for the neuroinfection of one patient with acute leukemia and disseminated aspergillosis [Citation262]. Patients’ underlying conditions included chronic granulomatous disease (CGD) [Citation263], diabetes mellitus [Citation264,Citation265], organ transplantation [Citation266–268], and hematologic malignancies [Citation265,Citation269]. Seven (58.3%) of 12 reported cases succumbed to death.
Mucormycosis may invade the CNS, typically as a result of intracranial extension of an adjacent paranasal sinus infection. The most common form of invasive disease is rhino-orbital-cerebral mucormycosis [Citation270]. To date, only about 30 cases of isolated CNS mucormycosis have been described in the literature [Citation271]. Isolated cerebral mucormycosis most frequently occurs in injection drug users [Citation272]. In a systematic review of mucormycosis in Iran in 2016, rhinocerebral mucormycosis was the most common clinical syndrome reported in 48 (50%) of 98 cases but no case of isolated cerebral mucormycosis was described [Citation273]. Several studies reported patients with rhino-cerebral or rhino-orbital-cerebral Mucormycosis [Citation273–279] but we found no study that described patients with isolated cerebral mucormycosis.
Dematiaceous fungi can cause a variety of infections in humans known as phaeohyphomycosis. The most severe forms of phaeohyphomycosis are infections of the CNS that mostly present as a brain abscess [Citation280]. Recently, an emerging number of patients with CNS infections due to dematiaceous fungi (cerebral phaeohyphomycosis) has been published in the literature, especially in organ transplant recipients [Citation281]. Cladophialophora bantiana accounted for about half of the cases in a review of 101 patients with cerebral phaeohyphomycosis [Citation281]. A few case reports described patients with cerebral phaeohyphomycosis in Iran [Citation282–285] mostly caused by Fonsecaea pedrosoi [Citation285], Nattrassia mangiferae [Citation282], Rhinocladiella mackenziei [Citation284], and Thielavia subthermophila [Citation283]. The underlying comorbidities of the reported patients were SLE, diabetes mellitus, and Behcet’s disease but it has been also reported in immunocompetent individuals.
Community-acquired CNS infections due to other fungal pathogens have been reported less frequently. A study that investigated deep-seated fungal infections in Iranian immunocompromised patients reported the isolation of candida species from CSF in five cases with Hodgkin lymphoma, Behcet’s disease, renal failure, history of nephrectomy, and acute lymphoblastic leukemia (ALL), C. neoformans from CSF in two cases with renal failure and diabetes mellitus, Aspergillus flavus from CSF of a kidney transplant patient, and Aspergillus fumigatus from brain abscess aspirate in a patient with malignancy and diabetes mellitus [Citation286]. However, the clinical syndromes of patients were not described. Trichosporon brain abscess has been also described in a 34-year-old man with a history of autoimmune hepatitis treated with corticosteroids [Citation287].
Parasitic CNS infections
Parasitic infestation of the CNS affects millions of people worldwide. While globalization causes a significant change in the geographic distribution of parasitic diseases, they occur mostly in the developing world [Citation288]. The main globally distributed parasitic diseases of the CNS include neurocysticercosis, echinococcosis (hydatid disease), malaria, toxoplasmosis, schistosomiasis, trypanosomiasis, toxocariasis, and onchocerciasis [Citation289].
Neurocysticercosis which is caused by the parasitic tapeworm Taenia solium is the most common parasitic disease of the human nervous system worldwide [Citation290]. Neurocysticercosis is not prevalent in Iran which could be related to the food habits and cultures of the Iranian population [Citation291]. To date, only one case of human neurocysticercosis has been reported which described a 63-year-old man in Tehran who finally succumbed to death due to healthcare-associated pneumonia [Citation292]. All other cases of Taenia solium infection in Iran were reported among domesticated pigs and wild animals. Accordingly, neurocysticercosis seems to be of little significance to human health in Iran [Citation293].
The echinococcal disease is caused by infection with the tapeworm Echinococcus, which belongs to the family Taeniidae. Among the four species of Echinococcus which produce infection in humans, E. granulosus, and E. multilocularis are the most common, causing cystic echinococcosis (CE) and alveolar echinococcosis (AE), respectively [Citation294]. CNS involvement of echinococcal disease is uncommon and most cases occur in the pediatric age group but it is responsible for a significant burden [Citation288]. Between 1990 and 2017, the pooled prevalence of CE and AE in Iran has been estimated at 5% and 2%, respectively, with a higher prevalence of CE in the North and West of the country [Citation295]. Notably, between 2000 and 2009, the overall annual cost of CE in Iran was estimated at US$232.3 million (95% CI US $103.1–397.8 million), including both direct and indirect costs [Citation296]. A systematic review of hydatid disease in Iran in 2019 identified 171 cases of brain involvement among 214,124 individuals with echinococcosis [Citation295]. The brain was the third most common organ involved, after the liver and lung, with a rate of 2.7%, in a report of 2199 cases of echinococcal disease who were operated on for the parasitic infection in Tehran, Iran [Citation297] (Supplementary Table 10).
Cerebral malaria is the most severe complication of infection caused by Plasmodium falciparum. Mortality is high (18–30%) [Citation298] and half of the worldwide annual deaths of malaria occur in those with cerebral malaria [Citation299]. Globally, cerebral malaria occurs primarily in African children and Asian adults, with the vast majority of cases occurring in children 5 years old or younger [Citation299]. Iran is one of the malaria-endemic countries in the world [Citation300]. During the last decades, malaria has been among the most important infectious diseases in Iran, with about 1500 cases per year, especially in Sistan-Baluchestan and Hormozgan Provinces. Between 2002 and 2017, 134,273 malaria cases were reported, with a decreasing incidence from 0.24/1000 cases in 2002 to 0.01/1000 in 2017 [Citation301]. In 2018, however, zero indigenous cases were reported for the first time [Citation302]. Despite the annual national incidence of malaria during the last decades, the actual burden of cerebral malaria in Iran is unclear. Our review found only two case reports of cerebral malaria from Iran in the literature [Citation303,Citation304].
T. gondii is another highly prevalent parasite in humans worldwide. Toxoplasmosis exerts a significant impact on healthcare services and individual healthcare costs. Earlier estimates, published in 2012, suggest that toxoplasmosis accounted for nearly $3 billion of illness-related costs and approximately 11,000 quality-adjusted life years (QALYs) lost per year [Citation305]. The seroprevalence of T. gondii varies substantially between the geographic regions throughout the world. The parasite is particularly more prevalent in Western European, South American, and African countries. In Iran, the overall seroprevalence of toxoplasmosis is relatively high, with a weighted overall prevalence of 37% (95% CI = 31–43) has been estimated in 2014 [Citation306]. The overall prevalence of anti-toxoplasma total antibody is higher among immunocompromised patients and is estimated as high as 50% [Citation307]. Cerebral toxoplasmosis is among the most prevalent forms of disease that is associated with significant morbidity and mortality [Citation305]. There are a few cases of cerebral toxoplasmosis described in a series of HIV-infected patients in Zahedan [Citation308] and Tehran [Citation309,Citation310] as well as case reports of HIV and non-HIV Iranian patients with CNS toxoplasmosis in the literature [Citation311–313] (Supplementary Table 10). However, the burden of neurological complications of toxoplasmosis in Iran is unclear.
Amebic meningoencephalitis is a rare CNS infection caused by free-living amoebae. It is a fatal condition, with a mortality rate of greater than 90% [Citation314]. Among the free-living amoebae, Naegleria fowleri, Paravahlkampfia francinae, Balamuthia mandrillaris, and species of Acanthamoeba and Sappinia have been identified as CNS pathogens [Citation315]. B. mandrillaris has been isolated from soil [Citation316] and hot springs [Citation317] in some parts of Iran and two cases of amoebic meningoencephalitis caused by Acanthamoeba species [Citation318] and N. fowleri [Citation319] have been reported in Yazd and Qom (both located in central Iran), respectively. The case of Acanthamoeba meningoencephalitis diagnosed postmortem in a 5-year-old immunocompetent girl [Citation320], and the case of Naegleria fowleri meningoencephalitis in a 5-month-old male infant [Citation321].
Toxocariasis is another zoonotic parasitic disease with a high impact on public health worldwide. It is a soil-transmitted nematode that can cause CNS involvement (i.e., neurotoxocariasis). It is an endemic parasitic infection in Iran. The pooled prevalence of human toxocariasis (2000–2017) has been estimated as high as 11% (95% CI 8–13%) while the pooled prevalence of Toxocara infection in dogs and cats is 17% (95% CI 14–20%) and 37% (95% CI 26–48%), respectively [Citation322]. In spite of the high prevalence of Toxocara infection in Iran, no report of neurotoxocariasis was found in the literature.
Schistosomiasis is also an important parasitic disease that has remained a major global public health concern worldwide [Citation323]. It is most prevalent in African countries, with more than 85% of reported cases occurring in these countries [Citation324]. Schistosomiasis is also considered an important infectious disease in EMR countries [Citation325]. In 2000, it was estimated that 4.17 million of the Iranian population were at risk of schistosomal infection, and 42,000 cases of infection caused by Schistosoma hematobiom were reported in the country. Despite reporting thousands of cases of schistosomal infection, Iran is not considered an endemic area for schistosomiasis [Citation326]. Khuzestan which is located in the Southwest of Iran is the only province of the country in which schistosomiasis has been reported [Citation327]. Implementing Iranian national programs for the prevention of Schistosomiasis, the infection has been eliminated in Iran [Citation328]. Neuroschistosomiasis is caused by granuloma formation around eggs that lodge in the CNS. Involvement of the brain (cerebral/encephalic schistosomiasis) often leads to seizures, whereas involvement of the spinal cord (spinal cord schistosomiasis or schistosomal myeloradiculopathy) can cause cord compression resulting in paralysis [Citation329]. We found no report of CNS involvement caused by this parasite published in Iran in the literature.
Several other infectious diseases, with a significant global burden of illness, particularly in low- and middle-income countries, such as leishmaniasis and trypanosomiasis can also involve the CNS.
Leishmaniasis is a parasitic vector-borne infection that is distributed in some parts of the New World and Old World. There are about 21 reported species of Leishmania that cause infections in humans and are endemic in the regions of Asia, Africa, America, and southern parts of Europe. Based on the species causing the infection, Leishmania infection in humans leads to three different forms of diseases: visceral, mucocutaneous, and cutaneous. Visceral leishmaniasis, also called Kala-Azar, is a chronic infection, associated with high mortality and morbidity [Citation330]. Although rare, neurological manifestations may occur with Kala-Azar [Citation331]. Peripheral neuropathy is probably the most common neurological manifestation of leishmaniasis but infrequent CNS involvement occurs and is closely related to the host immune status [Citation332]. In Iran, Leishmania infantum is the main etiological agent of Kala-azar. Kala-azar is endemic in southern and northwestern areas of Iran but it also has been reported sporadically in other parts of the country. The main reservoir hosts for L. infantum are domestic dogs and wild canines in various parts of Iran [Citation333]. CNS complications of Leishmaniasis have been very rarely reported in Iran. Sedaghattalab and Azizi reported a 54-year-old man from Yasuj in the Southwest of Iran with pons involvement by visceral leishmaniasis [Citation334].
Chagas disease still represents a major public health challenge in Latin America, where 8 to 10 million people are infected. It is not endemic in Iran or other countries in the WHO EMR [Citation335] however because of growing population movements, the disease has also spread to other continents [Citation336]. The involvement of the nervous system in Chagas’ disease is well established. CNS involvement is rare and prognosis depends on the patient’s age, as well as on the severity and location of the lesions. CNS involvement is very severe among children under 2 years of age, with a high fatality rate [Citation337].
Prion diseases
Prions are infectious proteins that cause neurodegenerative disorders [Citation338]. Sporadic Creutzfeldt-Jakob disease (CJD) is the most well-known prion disease that accounts for more than 90% of sporadic prion diseases [Citation339]. The spectrum of possible symptoms is highly heterogeneous and includes rapidly progressive dementia, cerebellar ataxia, and myoclonus [Citation340]. It is uniformly fatal and death usually occurs within one year of diagnosis [Citation341]. In 1996, a new form of acquired human prion disease was identified in the United Kingdom (UK), referred to as variant CJD (vCJD) [Citation342]. International CJD surveillance programs have been active since the emergence, in the mid-1990s, of vCJD [Citation343]. Following the first cases of vCJD described in the UK, cases of vCJD have been reported from several other countries. The majority of affected individuals had histories of extended residence in the UK or other Western European countries during the period (1980–96) of maximum global risk for human exposure to bovine spongiform encephalopathy [Citation344]. However, in a few cases, the exposure has been proposed to occur outside the UK and Western Europe, for example in Saudi Arabia [Citation344]. Thus, the possibility remains that other geographic foci of human infection exist, with potential for the future epidemics. There are rare reports of CNS diseases caused by prions in Iran [Citation345–347] which all describ patients with CJD however the actual incidence of this condition in the country is estimated to be higher. Considering that emerging animal prion disease might harbor the potential for zoonotic transmission to humans, CJD surveillance is a necessity at national and global levels to meet the potential for further cases of vCJD or the emergence of novel prion diseases in humans [Citation343].
HIV-associated CNS involvement
Before the introduction of effective combination antiretroviral therapy, approximately 10% of HIV-infected individuals initially presented with neurological disorders, and as HIV infection progressed to more advanced stages, 30–50% of them developed neurological complications [Citation348]. Two main groups of neurological disorders occur in association with HIV-1 infection: the opportunistic infections of the nervous system arising due to HIV-induced immunosuppression and the primary HIV-induced neurological syndromes, such as neurocognitive disorders [Citation349]. Furthermore, up to 17% of cases with primary HIV infection develop aseptic meningitis [Citation350]. In 2019, Iran’s HIV population was 59314, with an estimated 4,089 new infections occurring during the same period [Citation351]. However, information regarding the burden of neurological complications of HIV in Iran is unclear. We found only a few case series describing patients with HIV-associated neurological manifestations in the literature. In a series of 100 patients with acute encephalitis in Hamedan, Iran, one case of HIV-1 encephalitis was identified [Citation21]. Two single-center studies in Tehran described HIV-infected patients with neurological syndromes. In both studies, CNS Toxoplasmosis was the most common opportunistic infection of the nervous system in HIV-infected individuals. Other neuropathogens reported in HIV-infected patients were M. tuberculosis, JC virus, Nocardia species, and CMV [Citation309,Citation310]. Considering the high global burden of neurological complications associated with HIV infection, even in the antiretroviral therapy era [Citation352], regional and national surveillance of these serious disorders should be improved in Iran ().
Table 1. Infectious causes of CNS infection in Iran.
The impact of the coronavirus disease (COVID)-19 pandemic on the epidemiology & burden of CNS infection
The epidemiology of CNS infection is expected to change due to emerging pathogens, such as SARS-CoV-2. There are several reasons why SARS-CoV-2 may change the epidemiology of CNS infections and have a long-lasting impact on the burden of disease [Citation353]. First, neurological involvements are among the main complications of SARS-CoV-2 infection and occur more frequently in association with COVID-19 than influenza viruses or other respiratory tract infections [Citation353]. Second, the hygienic measures aimed at reducing the spread of COVID-19 have temporarily reduced the incidence of other aerogenic infectious diseases such as influenza infections and bacterial pneumonia [Citation354]. Following the reduction of the measures, increased incidences of respiratory infections have been observed [Citation355]. As baseline immunity to this disease has waned during COVID-19, these infections tend to be more severe and have worse outcomes. Third, immune-mediated neurological disorders have been reported in association with SARS-CoV-2 infection [Citation356] and following mass vaccination against SARS-CoV-2. Last but not least, there has been evidence of reactivation of other viruses in association with COVID-19 that might potentially affect the nervous system [Citation357]. Our systematic search included publications up to April 2020 which was shortly after the emergence of the COVID-19 pandemic in Iran and before the global awareness about its neurological complications. Thus, neurological complications associated with SARS-CoV-2 infection were not included in our review. However, a large number of recent literature on neurological infectious diseases in Iran and worldwide has focused on neurological manifestations of SARS-CoV-2 [Citation358–361]. Future studies should investigate how the COVID-19 pandemic influences on the epidemiology of CNS infections and their burden of illness in Iran and worldwide.
Surveillance of CNS infections in Iran
Surveillance is defined as the ongoing systematic collection, analysis, and interpretation of outcome-specific data for use in priority setting, policy decisions, planning, implementation, resource mobilization and allocation, and prediction and early detection of epidemics. The two key functions of a communicable disease surveillance system are early warning of potential threats to public health and program monitoring functions which may be disease-specific or multi-disease in nature. The early warning functions of surveillance are necessary for national, regional, and global health security [Citation362].
The surveillance forms an important cornerstone for the control of emerging and re-emerging infections [Citation363]. Neglected and (re)-emerging infectious diseases are a major public health concern in low- and middle-income countries, as they cause great morbidity and mortality [Citation11]. EMR is a hotspot for emerging and re-emerging infectious diseases [Citation67], particularly in some of the countries that share borders with Iran. Unstable political conditions and wars in some of Iran’s neighbors have resulted in immigrants and dislocations. Legal and illegal immigrants and refugees from those countries and international travels can be the source of some of the emerging and re-emerging infectious diseases in Iran [Citation174].
In Iran, the communicable disease surveillance system benefits from network-based and integrated health services, well-organized and knowledgeable personnel, national and international support, strategic and operational plans, and expanded programs of immunization [Citation364]. However, available evidence suggests that the occurrence of notifiable infectious diseases is seriously underreported and underestimated which is a cause for concern from a surveillance point of view. Several limitations have been proposed for the communicable disease surveillance system in Iran including disease-based case definition, poor public awareness, poor inter-sectoral and intra-sectoral collaboration and coordination, insufficient resources, lack of district laws or incomplete implementation of legislation, failure to provide feedback or ignoring for the results of the analysis, and lack of performance evaluation [Citation364–367]. Furthermore, the shift of health policies from communicable diseases to non-communicable diseases seems to be problematic for public health control strategies including effective surveillance activities for communicable diseases.
Meningitis, rabies, and acute flaccid paralysis are among the notifiable diseases in Iran. However, they are frequently underreported. This underreporting negatively impacts the effectiveness of the notification process as a ‘real-time’ surveillance tool and as an early warning system for outbreaks. Concerning the challenges that Iran face regarding emerging and re-emerging infectious diseases, improvement of the notification system for these serious infectious diseases is highly important to ensure proper health resource allocation and implementation of focused prevention and control strategies at both local and national level. Providing infrastructures with increased cooperation between various organizations to increase access to laboratory services in the form of an integrated network is also essential [Citation368].
Limitations
Our study has several limitations that should be mentioned. Most of the body of literature on CNS infections in Iran consists of case reports and a small retrospective case series from single centers. Therefore, we could not make a proper estimation of the incidence of different neurological syndromes associated with CNS infections and their burden of illnesses. However, we narratively reviewed the available data on this subject. Another important limitation was a high proportion of suspected CNS infections without a cause-specific diagnosis in the majority of studies. Furthermore, a large number of studies investigated CNS infections caused by one or small number of neuro-pathogens which made it difficult to conclude about the role of different causative pathogens in each neurological infectious syndrome. Future well-designed prospective cohort studies are required to address these issues.
Conclusion
Control and prevention of communicable diseases should be a top priority of health researchers, local health system managers, and policymakers. It needs a functional surveillance system on infectious disease to provide information, which can be used, for priority setting, policy decisions, planning, implementation, resource allocation, and prediction and early detection of epidemics.
CNS infections are among the most serious infectious diseases that are associated with significant morbidity and mortality. Our literature review showed that enteroviruses are the most commonly reported cause of viral meningitis, S. pneumoniae and Klebsiella species are the most common causes of bacterial and neonatal meningitis, respectively, and herpes simplex virus, the most common sporadic cause of viral encephalitis in Iran. Neurotuberculosis and neurobrucellosis place a huge burden on the healthcare system. HTLV-1 and hydatid disease are other notable infectious causes of neurological syndromes. Despite that, available data regarding many of the common global causes of CNS infections and their burden of illness is limited in Iran.
Improving the national surveillance system and implementing a nationwide data registry system for community-acquired CNS infections can provide precious information, which can be used, for dissemination of the data that enable public health authorities to optimize healthcare delivery and a cost-effective health strategy.
This information can also be used in understanding the microbial spectrum of endemic CNS infections and their burden of illness, optimizing their diagnostic and therapeutic strategies, and estimating the effectiveness of preventive measures in the country. In addition, effective surveillance activities for these serious disorders can detect outbreaks of epidemic-prone neuroinfections and monitor newly emerging and re-emerging neuropathogens.
Future perspective
CNS infections are among the most serious infectious diseases and neurological emergencies that are associated with significant morbidity and mortality. The epidemiology of CNS infections shows striking differences in geographic distribution. Currently, however, information regarding the epidemiology and precise estimates of the burden of disease are lacking for most neuroinfections in Iran, which limits preventative measures and targeted treatment. With increasing tourism and international travels, and unstable political conditions and wars in some of Iran’s neighbors that resulted in immigrants and dislocations, emerging and re-emerging neuropathogens have become an increasing challenge. Improving the national surveillance system and implementing a nationwide data registry system for CNS infections are necessary to provide updated information regarding the microbial spectrum and the burden of CNS infections to suggest optimal preventive, diagnostic, and therapeutic strategies.
Acute viral encephalitis
The most common cause of viral encephalitis in Iran and worldwide is herpes simplex virus encephalitis.
Arthropod-borne encephalitis viruses (arboviruses) are other notable causes of encephalitis. In Iran, West Nile virus is the most common arbovirus that has been identified in cases of viral encephalitis.
Information regarding the neurological complications of other arboviruses in Iran is lacking. This is despite the distribution of tick-borne encephalitis virus vectors in the Northern provinces of Iran and the emergence of Dengue and Chikungunya viruses in Iran.
Although Iran received a certificate for measles elimination in October 2019, cross-border travel from Afghanistan and Pakistan as well as the growing vaccine hesitancy pose the risk of a resurgence of measles and its neurological complications.
Rabies has been distributed in all provinces of Iran. Despite national efforts to control this deadly neuroinfection, Iran faces many challenges in the elimination of rabies and it is still a public health concern in the country.
Viral meningitis
In Iran, enteroviruses are the most common cause of viral meningitis reported in the literature. Following the elimination of poliovirus infections in the majority of countries, non-polio enteroviruses are emerging as the causative agents of CNS involvement in Iran and worldwide.
Acute bacterial meningitis
Following the introduction of H. influenzae type b childhood vaccination in Iran in 2014, a substantial decrease has occurred in the overall incidence and mortality of bacterial meningitis but it is still a great concern.
Although Streptococcus agalactiae is the main cause of neonatal meningitis in developed countries, Klebsiella species has been reported as the most common causative pathogen of neonatal meningitis in Iran. Studies published on this subject are however scarce.
Chronic meningitis
The causes of chronic meningitis vary between countries and regions. Globally, tuberculous and cryptococcal meningitis are the two most common types of chronic meningitis but in Iran, M. tuberculosis and Brucella species are the predominant causes of chronic meningitis.
Brain abscess
Consistent with previous literature, limited evidence reported oral cavity bacteria such as Streptococcus viridans as the most common cause of brain abscesses in Iran.
Infectious myelitis
In the post-polio eradication era, non-polio enteroviruses have been reported as the main cause of acute flaccid paralysis in Iran, with Echovirus 3 being the most common isolated serotype.
HTLV-1-associated myelopathy/tropical spastic paraparesis is an important cause of infectious myelopathy which causes a major health problem in some parts of Iran.
CNS infections caused by other intracellular bacterial pathogens
Despite a decreasing trend in the overall incidence of tuberculosis in Iran, available data suggest that the Iranian population is greatly affected by tuberculosis. Neurotuberculosis is estimated to be associated with a substantial burden of illness in Iran, particularly in adults. Literature on this subject is however limited. BCG vaccination coverage has been part of the Iranian neonatal vaccination program since 1984 and has reduced the risk of tuberculosis and the development of meningeal and miliary tuberculosis in children.
Human brucellosis remains a huge burden in Iran, with an increasing trend in incidence over the last few years. Neurobrucellosis accounts for 0.2–8% of cases who are admitted to the hospital due to a variety of complications associated with brucellosis in the country.
Although available literature highlighted the presence of Listeria species. in a variety of raw and ready-to-eat food samples in Iran, information regarding the overall burden of human listeriosis or its neurological involvement is scares.
CNS infections caused by spirochetes
In Iran, the overall prevalence of syphilis is relatively low but it is more prevalent among high-risk groups. Available data regarding the burden of neurosyphilis in Iran, however, is limited.
Despite the endemicity of tick-borne relapsing fever and leptospirosis in Iran, the burden of neurological complications caused by these pathogens is unclear.
Although Lyme borreliosis is the most prevalent tick-borne zoonosis worldwide, it is not endemic in Iran.
Fungal CNS infections
Cryptococcus neoformans, the most common cause of fungal meningitis worldwide, has been isolated from birds’ excreta, trees, and soil in different areas of Iran, but published literature about cryptococcal meningitis is limited to a few case reports and small case series.
Parasitic CNS Infections
Echinococcal disease, malaria, toxocariasis, and toxoplasmosis are endemic in Iran however information about neurological complications caused by these pathogens is limited.
Neurocysticercosis which is the most common parasitic disease of the human nervous system worldwide, seems to be of little significance to human health in Iran.
Surveillance of CNS infections in Iran
Available evidence suggests that the occurrence of some notifiable infectious diseases such as meningitis is seriously underestimated in Iran which is a cause for concern from a surveillance point of view. This underreporting negatively impacts the effectiveness of the notification process as a ‘real-time’ surveillance tool and as an early warning system for outbreaks for proper health resource allocation and implementation of focused prevention and control strategies at both local and national levels.
Conclusion
Improving the national surveillance system and implementing a nationwide data registry system for CNS infections are necessary to provide updated information regarding the microbial spectrum and the burden of CNS infections to suggest optimal preventive, diagnostic, and therapeutic strategies.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Supplemental Table 1
Download MS Word (318.4 KB)Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fnl-2023-0017
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Erdem H , Inan A , Guven E et al. The burden and epidemiology of community-acquired central nervous system infections: a multinational study. Eur. J. Clin. Microbiol. Infect. Dis. 36(9), 1595–1611 (2017).
- Van de Beek D , de Gans J , Tunkel AR et al. Community-acquired bacterial meningitis in adults. N. Engl. J. Med. 354(1), 44–53 (2006).
- van de Beek D , de Gans J , Tunkel AR et al. Community-Acquired Bacterial Meningitis in Adults. N. Engl. J. Med. 354(1), 44–53 (2006).
- Dalman C , Allebeck P , Gunnell D et al. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am. J. Psychiatry 165(1), 59–65 (2008).
- Lotz SK , Blackhurst BM , Reagin KL et al. Microbial infections are a risk factor for neurodegenerative diseases. Front. Cell Neurosci. 15, 691136 (2021).
- Sousa Junior IP , Vieira TCRG . Enterovirus infection and its relationship with neurodegenerative diseases. Memórias do Instituto Oswaldo Cruz. 118, e220252 (2023).
- Robertson FC , Lepard JR , Mekary RA et al. Epidemiology of central nervous system infectious diseases: a meta-analysis and systematic review with implications for neurosurgeons worldwide. J. Neurosurg. 130(4), 1–20 (2018).
- Robertson FC , Lepard JR , Mekary RA et al. Epidemiology of central nervous system infectious diseases: a meta-analysis and systematic review with implications for neurosurgeons worldwide. J. Neurosurg. 130(4), 1107–1126 (2018).
- Bennett JE , Dolin R , Blaser MJ . Mandell, Douglas, and Bennett’s principles and practice of infectious diseases (9th ed.). Elsevier Saunders (2019).
- John CC , Carabin H , Montano SM et al. Global research priorities for infections that affect the nervous system. Nature 527(7578), S178–S186 (2015).
- Cárdenas G , Salgado P , Laura-Foronda E et al. Neglected and (re-)emergent infections of the CNS i n low-/middle-income countries. Le Infezioni in Medicina 29(4), 513–525 (2021).
- Ghiami-Shamami F , Sabziparvar AA , Shinoda S . Long-term comparison of the climate extremes variability in different climate types located in coastal and inland regions of Iran. Theoretical and Applied Climatology 136(3–4), 875–897 (2019).
- Ashraf S , Yazdani-Biouki R , Mousavi-Baygi M et al. Investigation of temporal and spatial climate variability and aridity of Iran. Theoretical and Applied Climatology 118, 35–46(2014).
- Ghasemi AR , Khalili D . The association between regional and global atmospheric patterns and winter precipitation in Iran. Atmospheric Research 88(2), 116–133 (2008).
- Wang H , Zhao S , Wang S et al. Global magnitude of encephalitis burden and its evolving pattern over the past 30 years. The Journal of Infection 84(6), 777–787 (2022).
- Venkatesan A , Michael BD , Probasco JC et al. Acute encephalitis in immunocompetent adults. The Lancet 393(10172), 702–716 (2019).
- Tyler KL . Acute Viral Encephalitis. N. Engl. J. Med. 379(6), 557–566 (2018).
- Gluckman SJ . Viral encephalitis in adults (2021). https://www.uptodate.com/contents/viral-encephalitis-in-adults?search=HSV%20ENCEPHALITIS%20ADULTS&source=search_result&selectedTitle=3∼96&usage_type=default&display_rank=3
- Sheybani F , Arabikhan H , Naderi H . Herpes simplex encephalitis (HSE) and its outcome in the patients who were admitted to a tertiary care hospital in Mashhad, Iran, over a 10-year period. Journal of Clinical and Diagnostic Research: JCDR 7(8), 1626 (2013).
- Sasan MS , Jarahi L . Epidemiology Of Pediatrics Encephalitis in Mashhad. Medical Journal of Mashhad University of Medical Sciences 60(3), 503–509 (2017).
- Ghannad MS , Solgi G , Hashemi SH et al. Herpes simplex virus encephalitis in hamadan, iran. Iranian Journal of Microbiology 5(3), 272 (2013).
- Peidaee E , Sheybani F , Naderi H et al. The etiological spectrum of febrile encephalopathy in adult patients: a cross-sectional study from a developing country. Emergency Medicine International 2018, (2018).
- Hashemian S , Ashrafzadeh F , Akhondian J et al. Epstein-barr virus encephalitis: a case report. Iranian Journal of Child Neurology 9(1), 107–110 (2015).
- Petersen LR . Arthropod-borne encephalitides (2021). https://www.uptodate.com/contents/arthropod-borne-encephalitides?search=Arthropod-borne%20encephalitides&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1
- Madewell ZJ . Arboviruses and their vectors. Southern Medical Journal 113(10), 520 (2020).
- Bogovic P , Strle F . Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World Journal of Clinical Cases 3(5), 430–441 (2015).
- Petersen L . Epidemiology and pathogenesis of West Nile virus infection (2021). https://www.uptodate.com/contents/epidemiology-and-pathogenesis-of-west-nile-virus-infection?search=Epidemiology%20and%20pathogenesis%20of%20West%20Nile%20virus%20infection&source=search_result&selectedTitle=1~90&usage_type=default&display_rank=1
- McDonald E , Mathis S , Martin SW et al. Surveillance for West Nile Virus Disease—United States, 2009–2018. Wiley Online Library, 1959–1974 (2021).
- Eybpoosh S , Fazlalipour M , Baniasadi V et al. Epidemiology of West Nile Virus in the Eastern Mediterranean region: a systematic review. PLoS Neglected Tropical Diseases 13(1), e0007081 (2019).
- Naficy K , Saidi S . Serological survey on viral antibodies in Iran. Trop. Geogr. Med. 22(2), 183–188 (1970).
- Chinikar S , Ghiasi SM , Shah-Hosseini N et al. Preliminary study of dengue virus infection in Iran. Travel Medicine and Infectious Disease 11(3), 166–169 (2013).
- Bagheri M , Terenius O , Oshaghi MA et al. West Nile Virus in Mosquitoes of Iranian Wetlands. Vector Borne and Zoonotic Diseases (Larchmont, NY) 15(12), 750–754 (2015).
- Ahmadnejad F , Otarod V , Fallah MH et al. Spread of West Nile virus in Iran: a cross-sectional serosurvey in equines, 2008–2009. Epidemiol. Infect. 139(10), 1587–1593 (2011).
- Saidi S , Tesh R , Javadian E et al. The prevalence of human infection with West Nile virus in Iran. Iranian Journal of Public Health 5(1), 8–13 (1976).
- Sharifi Z , Mahmoodian Shooshtari M , Talebian A . A study of West Nile virus infection in Iranian blood donors. Archives of Iranian Medicine 13(1), 1–4 (2010).
- Chinikar S , Shah-Hosseini N , Mostafavi E et al. Seroprevalence of West Nile virus in Iran. Vector Borne and Zoonotic Diseases (Larchmont, NY) 13(8), 586–589 (2013).
- Ziyaeyan M , Behzadi MA , Leyva-Grado VH et al. Widespread circulation of West Nile virus, but not Zika virus in southern Iran. PLoS Neglected Tropical Diseases 12(12), e0007022 (2018).
- Meshkat Z , Chinikar S , Shakeri M et al. Prevalence of West Nile virus in Mashhad, Iran: a population–based study. Asian Pacific Journal of Tropical Medicine 8(3), 203–205 (2015).
- Chinikar S , Javadi A , Ataei B et al. Detection of West Nile virus genome and specific antibodies in Iranian encephalitis patients. Epidemiology & Infection 140(8), 1525–1529 (2012).
- World Health Organization . Tick-borne encephalitis. World Health Organization (2014). www.who.int/immunization/diseases/tick_encephalitis/en/
- Erdem H , Inan A , Guven E et al. The burden and epidemiology of community-acquired central nervous system infections: a multinational study. European Journal of Clinical Microbiology & Infectious Diseases 36(9), 1595–1611 (2017).
- Bogovič P , Strle F . Tick-Borne Encephalitis. Meningoencephalitis-Disease Which Requires Optimal Approach in Emergency Manner. IntechOpen (2017).
- Süss J . Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia—an overview. Ticks and Tick-Borne Diseases 2(1), 2–15 (2011).
- Rabiee MH , Mahmoudi A , Siahsarvie R et al. Rodent-borne diseases and their public health importance in Iran. PLoS Neglected Tropical Diseases 12(4), 3–10 (2018).
- Bogovic P , Strle F . Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World Journal of Clinical Cases: WJCC 3(5), 430 (2015).
- Turtle L , Solomon T . Japanese encephalitis — the prospects for new treatments. Nature Reviews Neurology 14(5), 298–313 (2018).
- Vannice KS , Hills SL , Schwartz LM et al. The future of Japanese encephalitis vaccination: expert recommendations for achieving and maintaining optimal JE control. NPJ Vaccines 6(1), 82 (2021).
- World Health Organization . Japanese encephalitis reported cases. World Health Organization (2019). https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencejapenc.html
- Lum L , Ng CJ , Khoo EM . Managing dengue fever in primary care: a practical approach. Malaysian Family Physician: the Official Journal of the Academy of Family Physicians of Malaysia 9(2), 2–10 (2014).
- Kulkarni R , Pujari S , Gupta D . Neurological Manifestations of Dengue Fever. Annals of Indian Academy of Neurology 24(5), 693–702 (2021).
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. World Health Organization, Geneva (2009). www.ncbi.nlm.nih.gov/books/NBK143159/?report=classic
- Verma R , Sahu R , Holla V . Neurological manifestations of dengue infection: a review. J. Neurol. Sci. 346(1–2), 26–34 (2014).
- El-Hefni A . Recent Outbreaks of Dengue Fever in the WHO Eastern Mediterranean Region: A Narrative Mini-review. Journal of African Research and Studies and the Nile Basin 1(2), 224–241 (2020).
- Heydari M , Metanat M , Rouzbeh-Far MA et al. Dengue Fever as an Emerging Infection in Southeast Iran. The American Journal of Tropical Medicine and Hygiene 98(5), 1469–1471 (2018).
- Brizzi K . Neurologic Manifestation of Chikungunya Virus. Current Infectious Disease Reports 19(2), 6 (2017).
- Petersen LR , Powers AM . Chikungunya: epidemiology. F1000Research 5, 2–3 (2016).
- Malik MR , Mnzava A , Mohareb E et al. Chikungunya outbreak in Al-Hudaydah, Yemen, 2011: epidemiological characterization and key lessons learned for early detection and control. Journal of Epidemiology and Global Health 4(3), 203–211 (2014).
- El Bushra HE , Habtewold BW , Al Gasseer N et al. Outbreak of chikungunya fever in Sudan, 2018–2019. Juniper Online J. Public Health 4, 555644 (2019).
- Bakhshi H , Mousson L , Moutailler S et al. Detection of arboviruses in mosquitoes: evidence of circulation of chikungunya virus in Iran. PLoS Neglected Tropical Diseases 14(6), e0008135 (2020).
- Pouriayevali MH , Rezaei F , Jalali T et al. Imported cases of Chikungunya virus in Iran. BMC Infectious Diseases 19(1), 1004 (2019).
- Petersen LR , Jamieson DJ , Powers AM et al. Zika Virus. N. Engl. J. Med. 374(16), 1552–1563 (2016).
- World Health Organization . Zika virus. World Health Organization (2018). www.who.int/news-room/fact-sheets/detail/zika-virus
- Hasbun R . Meningitis and Encephalitis: Management and Prevention Challenges. Springer, Switzerland (2018).
- Ziyaeyan M , Behzadi MA , Leyva-Grado VH et al. Widespread circulation of West Nile virus, but not Zika virus in southern Iran. PLoS Neglected Tropical Diseases 12(12), e0007022 (2018).
- Gans H , Maldonado YA , Post T . Measles: Clinical manifestations, diagnosis, treatment, and prevention (2022). https://www.uptodate.com/contents/measles-clinical-manifestations-diagnosis-treatment-and-prevention?search=MEASLES&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1
- Gans H , Maldonado YA , Post T . Measles: Clinical manifestations, diagnosis, treatment, and prevention (2022). https://www.uptodate.com/contents/measles-clinical-manifestations-diagnosis-treatment-and-prevention?search=MEASLES&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1
- Mostafavi E , Ghasemian A , Abdinasir A et al. Emerging and re-emerging infectious diseases in the WHO Eastern Mediterranean region, 2001–2018. International Journal of Health Policy and Management 11(8), 1286 (2022).
- Namaki S , Gouya MM , Zahraei SM et al. The elimination of measles in Iran. The Lancet Global Health 8(2), e173–e174 (2020).
- Shafyi A , Lotfi J , Mirchamsy H . Subacute sclerosing panencephalitis in Iran. The Kitasato Archives of Experimental Medicine 57(4), 267–271 (1984).
- Patterson MC . Neurological Complications of Measles (Rubeola). Current Neurology and Neuroscience Reports 20(2), 2 (2020).
- Roberts L . How COVID hurt the fight against other dangerous diseases. Nature 592(7855), 502–504 (2021).
- Blanton JD , Dyer J , McBrayer J et al. Rabies surveillance in the United States during 2011. J. Am. Vet. Med. Assoc. 241(6), 712–722 (2012).
- World Health Organization . WHO expert consultation on rabies: third report. World Health Organization (2018).
- Abedi M , Doosti-Irani A , Jahanbakhsh F et al. Epidemiology of animal bite in Iran during a 20-year period (1993–2013): a meta-analysis. Tropical Medicine and Health 47(1), 55 (2019).
- Hosseini Heydarabadi F , Baessi K , Bashar R et al. A phylogenetic study of new rabies virus strains in different regions of Iran. Virus Genes 56(3), 361–368 (2020).
- Khashei B , Baferani MA , Bashar R et al. Ante-mortem Diagnosis of Human Rabies Cases Using SYBR Green Real-Time PCR. Archives of Iranian Medicine (AIM) 21(10), 473–477 (2018).
- Ebrahimzadeh Leylabadlo H , Bannazadeh Baghi H . Rabies Elimination by 2030: What Challenges Does Iran Face? Iranian Journal of Public Health 49(7), 1397–1398 (2020).
- Goenka A , Michael BD , Ledger E et al. Neurological manifestations of influenza infection in children and adults: results of a National British Surveillance Study. Clin. Infect. Dis. 58(6), 775–784 (2014).
- Davis LE . Neurologic and muscular complications of the 2009 influenza A (H1N1) pandemic. Current Neurology and Neuroscience Reports 10(6), 476–483 (2010).
- Gouya M , Nabavi M , Soroush M et al. Mortality from pandemic influenza A (H1N1) in Iran. Iranian Red. Crescent Medical Journal 13(10), 698 (2011).
- Asadi-Pooya AA , Yaghoubi E , Nikseresht A et al. The Neurological Manifestations of H1N1 Influenza Infection; Diagnostic Challenges and Recommendations. Iranian Journal of Medical Sciences 36(1), 36–39 (2011).
- Logan SAE , MacMahon E . Viral meningitis. BMJ 336(7634), 36–40 (2008).
- Delerme S , Castro S , Viallon A et al. Meningitis in elderly patients. European Journal of Emergency Medicine 16(5), 273–276 (2009).
- Cantu RM , M Das J . Viral Meningitis. StatPearls. StatPearls Publishing, Treasure Island (FL) (2023).
- Mathew S , Al Khatib HA , Al Ansari K et al. Epidemiology Profile of Viral Meningitis Infections Among Patients in Qatar (2015–2018). Frontiers in Medicine 8, 663694 (2021).
- Logan SAE , MacMahon E . Viral meningitis. BMJ 336(7634), 36–40 (2008).
- Kupila L , Vuorinen T , Vainionpää R et al. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology 66(1), 75–80 (2006).
- Hosseininasab A , Alborzi A , Ziyaeyan M et al. Viral etiology of aseptic meningitis among children in southern Iran. J. Med. Virol. 83(5), 884–888 (2011).
- Rahimi P , Naser HM , Siadat SD et al. Genotyping of human parechoviruses in Iranian young children with aseptic meningitis and sepsis-like illness. J. Neurovirol. 19(6), 595–600 (2013).
- Rasti M , Makvandi M , Neisi N et al. Three cases of mumps virus and enterovirus coinfection in children with enteroviral meningitis. Medicine 95(49), e5610 (2016).
- Ghabouli Shahroodi MJ , Ghazvini K , Sadeghi R et al. Enteroviral Meningitis in neonates and children of Mashhad, Iran. Jundishapur Journal of Microbiology 9(5), 2–3 (2016).
- Cecilia Di Pentima M , Kaplan SL . Viral meningitis: epidemiology, pathogenesis, and etiology in children (2022). https://www.uptodate.com/contents/viral-meningitis-epidemiology-pathogenesis-and-etiology-in-children?search=viral%20meningitis&topicRef=6028&source=see_link
- Rahimi P , Roohandeh A , Sohrabi A et al. Impact of Human Enterovirus 71 Genotypes in Meningoencephalitis in Iran. Jundishapur J. Microbiol. 8(12), e27113 (2015).
- Roohandeh A , Rahimi P , Sohrabi A et al. Frequency of human enterovirus 71 in children under 8 years old with aseptic menengitis in Tehran. Clinical Laboratory 59(7–8), 915–920 (2013).
- Hasbun R , Rosenthal N , Balada-Llasat JM et al. Epidemiology of Meningitis and Encephalitis in the United States, 2011–2014. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 65(3), 359–363 (2017).
- Martin NG , Iro MA , Sadarangani M et al. Hospital admissions for viral meningitis in children in England over five decades: a population-based observational study. The Lancet Infectious Diseases 16(11), 1279–1287 (2016).
- Zahraei SM , Gouya MM , Mokhtari Azad T et al. Successful control and impending elimination of measles in the Islamic Republic of Iran. The Journal of Infectious Diseases 204(Suppl. 1), S305–S311 (2011).
- Esteghamati A , Keshtkar A , Heshmat R et al. Adverse reactions following immunization with MMR vaccine in children at selected provinces of Iran. Archives of Iranian Medicine 14(2),91–95 (2011).
- Sasan MS , Haji Shamsaei M . Aseptic Meningitis after MMR Vaccination in Children of Mashhad. Medical Journal of Mashhad University of Medical Sciences 53(3), 163–168 (2010).
- Oskouie Sh A , Barzegar M , Malekian A . Relationship between aseptic meningitis and MMR vaccination. Medical Journal of Tabriz University of Medical Sciences 28(4), 81–84 (2010).
- Pormohammad A , Behboudi E , Ramezani A et al. Global Study of Viral Meningitis: A Systematic Review and Meta-analysis. International Journal of Pediatrics 10(4), 15865–15880 (2022).
- Pormohammad A , Lashkarbolouki S , Azimi T et al. Clinical characteristics and molecular epidemiology of children with meningitis in Tehran, Iran: a prospective study. New Microbes and New Infections 32, 100594 (2019).
- Akya A , Ahmadi K , Zehtabian S et al. Study of the frequency of herpesvirus infections among patients suspected aseptic meningitis in the West of Iran. Jundishapur Journal of Microbiology 8(10), 3–4 (2015).
- Sasan MS , Alborzi A , Ziyaeyan M . Epidemiology of aseptic meningitis in infants and children (shiraz-Iran). Archives of Clinical Infectious Diseases 7(4), 116–118 (2012).
- Amjadi O , Rafiei A , Haghshenas M et al. A systematic review and meta-analysis of seroprevalence of varicella zoster virus: a nationwide population-based study. Journal of Clinical Virology 87, 49–59 (2017).
- Allami A , Mohammadi N . Varicella immunity in Iran: an age-stratified systematic review and meta-analysis. Iranian Journal of Microbiology 6(6), 372–381 (2014).
- Bennett JE , Dolin R , Blaser MJ . Mandell, Douglas, and Bennett’S Principles and Practice of Infectious Diseases (9th ed.). Elsevier, Philadelphia, PA, 19103–2899 (2020).
- Ebrahimi TF , Noorbakhsh S , Monavari HR et al. Iranian Journal of Pathology, SEARCHING the human herpes virus 6 and 7 (PCR) in CSF of children admitted in Pediatric Ward of Rasoul Hospital, Tehran, Iran. 7(2), 107–111 (2012).
- Sanaei Dashti A , Alizadeh S , Karimi A et al. Diagnostic value of lactate, procalcitonin, ferritin, serum-C-reactive protein, and other biomarkers in bacterial and viral meningitis: a cross-sectional study. Medicine 96(35), e7637 (2017).
- Bonthius DJ . Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic disease in the fetus, child, and adult. Semin. Pediatr. Neurol. 19(3), 89–95 (2012).
- Reed KD . Viral Zoonoses. Reference Module in Biomedical Sciences. Elsevier (2018).
- Dehghani R , Kassiri H , Khodkar I et al. A comprehensive overview on sandfly fever. Journal of Acute Disease 10(3), 98 (2021).
- Saidi S , Tesh R , Javadian E et al. Studies on the epidemiology of sandfly fever in Iran. II. The prevalence of human and animal infection with five phlebotomus fever virus serotypes in Isfahan province. The American Journal of Tropical Medicine and Hygiene 26(2), 288–293 (1977).
- Tesh R , Saidi S , Javadian E et al. Studies on the epidemiology of sandfly fever in Iran. I. Virus isolates obtained from Phlebotomus. The American Journal of Tropical Medicine and Hygiene 26(2), 282–287 (1977).
- Shiraly R , Khosravi A , Farahangiz S . Seroprevalence of sandfly fever virus infection in military personnel on the western border of Iran. Journal of Infection and Public Health 10(1), 59–63 (2017).
- Zunt JR , Kassebaum NJ , Blake N et al. Global, regional, and national burden of meningitis, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology 17(12), 1061–1082 (2018).
- Brouwer MC , Van De Beek D . Epidemiology of community-acquired bacterial meningitis. Current Opinion in Infectious Diseases 31(1), 78–84 (2018).
- Esteghamati A , Asgari F , Goodarzi N . National Guideline on Meningitis Surveillance in Iran (3rd ed.). The Ministry of Health and Medical Education of Iran, Tehran, Iran (2004).
- Giorgi Rossi P , Mantovani J , Ferroni E et al. Incidence of bacterial meningitis (2001–2005) in Lazio, Italy: the results of a integrated surveillance system. BMC Infectious Diseases 9(1), 13 (2009).
- Soeters HM , Diallo AO , Bicaba BW et al. Bacterial Meningitis Epidemiology in Five Countries in the Meningitis Belt of Sub-Saharan Africa, 2015–2017. The Journal of Infectious Diseases 220(Suppl. 4), S165–S174 (2019).
- Mosavi-Jarrahi A , Esteghamati A , Asgari F et al. Temporal analysis of the incidence of meningitis in the Tehran metropolitan area, 1999–2005. Population Health Metrics 7, 19 (2009).
- Alizadeh Chamkhaleh M , Esteghamati A , Sayyahfar S et al. Serotype distribution of Streptococcus pneumoniae among healthy carriers and clinical patients: a systematic review from Iran. European Journal of Clinical Microbiology & Infectious Diseases 39(12), 2257–2267 (2020).
- Attarpour-Yazdi MM , Ghamarian A , Mousaviehzadeh M et al. Identification of the serotypes of bacterial meningitis agents; implication for vaccine usage. Iranian Journal of Microbiology 6(4), 211 (2014).
- Heidari S , Karami M , Zahraei SM et al. Epidemiological profile of meningitis following pentavalent vaccination in Iran: impact of vaccine introduction. Journal of Epidemiology and Global Health 11(3), 310 (2021).
- Tavana AM , Ataee R . Meningococcal meningitis control in Iran: five year comparative study 2000–2004. Journal of Medical Sciences (Pakistan) 9(1), 51–54 (2009).
- Mehrdadi S . Acute Bacterial Meningitis: Diagnosis, Treatment and Prevention. J. Arch. Mil. Med. 6(4), e84749 (2019).
- van der Flier M . Neonatal meningitis: small babies, big problem. The Lancet Child & Adolescent Health 5(6), 386–387 (2021).
- Furyk J , Swann O , Molyneux E . Systematic review: neonatal meningitis in the developing world. Tropical Medicine & International Health 16(6), 672–679 (2011).
- Zamani A , Zamani F . Cerebrospinal fluid findings in neonatal bacterial meningitis. Medical Journal of The Islamic Republic of Iran (MJIRI) 19(3), 241–245 (2005).
- Khalessi N , Afsharkhas L . Neonatal meningitis: risk factors, causes, and neurologic complications. Iranian Journal of Child Neurology 8(4), 46 (2014).
- Aletayeb MH , Ahmad FS , Masood D . Eleven-year study of causes of neonatal bacterial meningitis in Ahvaz, Iran. Pediatrics International 52(3), 463–466 (2010).
- Aletayeb MH , Ahmad FS , Masood D . Eleven-year study of causes of neonatal bacterial meningitis in Ahvaz, Iran. Pediatrics International: Official Journal of the Japan Pediatric Society 52(3), 463–466 (2010).
- Shiva F , Mosaffa N , Khabbaz R et al. Lumbar puncture in neonates under and over 72 hours of age. Journal of the College of Physicians and Surgeons--Pakistan: JCPSP 16(8), 525–528 (2006).
- Zelellw DA , Dessie G , Worku Mengesha E et al. A systemic review and meta-analysis of the leading pathogens causing neonatal sepsis in developing countries. BioMed Research International 2021, 1–20 (2021).
- Brouwer MC , Tunkel AR , van de Beek D . Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin. Microbiol. Rev. 23(3), 467–492 (2010).
- Berangi Z , Karami M , Mohammadi Y et al. Epidemiological profile of meningitis in Iran before pentavalent vaccine introduction. BMC Pediatrics 19(1), 370 (2019).
- van de Beek D , Cabellos C , Dzupova O et al. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clinical Microbiology and Infection 22, S37–S62 (2016).
- Marandi FR , Rahbar M , Sabourian R et al. Evaluation of Iranian microbiology laboratories for identification of etiologic agents of bacterial meningitidis. Survey results of an external quality assessment scheme (EQAS) programme. JPMA: The Journal of the Pakistan Medical Association 60(1), 48–51 (2010).
- Houri H , Pormohammad A , Riahi SM et al. Acute bacterial meningitis in Iran: systematic review and meta-analysis. PLOS ONE 12(2), e0169617 (2017).
- Ghotaslou R , Yeganeh-Sefidan F , Salahi-Eshlaqi B et al. Etiology of Acute Bacterial Meningitis in Iran: a Systematic Review. Acta Medica Iranica 53(8), 454–461 (2015).
- Motamedifar M , Ebrahim-Saraie HS , Mansury D et al. Prevalence of etiological agents and antimicrobial resistance patterns of bacterial meningitis in Nemazee Hospital, Shiraz, Iran. Archives of Clinical Infectious Diseases 10(2), e22703 (2015).
- Abdinia B , Rezaee MA , Oskouie SA . Etiology and antimicrobial resistance patterns of acute bacterial meningitis in children: a 10-year referral hospital-based study in northwest iran. Iranian Red Crescent Medical Journal 16(7), e17616 (2014).
- Heydari B , Khalili H , Karimzadeh I et al. Clinical, paraclinical, and antimicrobial resistance features of community-acquired acute bacterial meningitis at a large infectious diseases ward in Tehran, Iran. Iranian Journal of Pharmaceutical Research: IJPR 15(1), 347 (2016).
- Haghi-Ashtiani MT , Mamishi S , Shayanfar N et al. Antimicrobial susceptibility profiles associated with bacterial meningitis among children: a referral hospital-based study in Iran. Acta Microbiologica et Immunologica Hungarica 58(4), 273–278 (2011).
- Rezaeizadeh G , Pourakbari B , Ashtiani MH et al. Antimicrobial susceptibility of bacteria isolated from cerebrospinal fluids in an Iranian referral pediatric center, 1998–2008. Maedica 7(2), 131 (2012).
- Bokaeian M , Khazaei HA , Javadimehr M . Serotype distribution and antimicrobial resistance of invasive Streptococcus pneumoniae isolates from children in Zahedan, Iran. African Journal of Microbiology Research 6(1), 28–33 (2012).
- Houri H , Tabatabaei SR , Saee Y et al. Distribution of capsular types and drug resistance patterns of invasive pediatric Streptococcus pneumoniae isolates in Teheran, Iran. International Journal of Infectious Diseases 57, 21–26 (2017).
- Tunkel AR . Chronic meningitis. Current Infectious Disease Reports 1(2), 160–165 (1999).
- Coyle PK . Chronic meningitis. Current Treatment Options in Neurology 2(4), 375–387 (2000).
- Anderson N , Willoughby E . Chronic meningitis without predisposing illness—a review of 83 cases. QJM: An International Journal of Medicine 63(1), 283–295 (1987).
- Helbok R , Pongpakdee S , Yenjun S et al. Chronic meningitis in Thailand. Neuroepidemiology 26(1), 37–44 (2006).
- Lan SH , Chang WN , Lu CH et al. Cerebral infarction in chronic meningitis: a comparison of tuberculous meningitis and cryptococcal meningitis. QJM: An International Journal of Medicine 94(5), 247–253 (2001).
- Erdem H , Inan A , Guven E et al. The burden and epidemiology of community-acquired central nervous system infections: a multinational study. European Journal of Clinical Microbiology & Infectious Diseases 36(9), 1595–1611 (2017).
- Bineshfar N , Rezaei A , Mirahmadi A et al. Evaluation of the epidemiologic, clinical, radiologic, and treatment methods of patients with subacute and chronic meningitis. BMC Neurology 22(1), 340 (2022).
- Ahmadinejad Z , Rasolinejad M et al. Chronic Meningitis: a study on epidemiological and clinical findings, treatment results and prognosis of 97 patients. Acta Medica Iranica 39(4), 185–190 (1970).
- Bennett JE , Dolin R , Blaser MJ . Mandell, Douglas, and Bennett’s principles and practice of infectious diseases (9th ed.). Elsevier Inc. (2020).
- Brouwer MC , van de Beek D . Epidemiology, diagnosis, and treatment of brain abscesses. Current Opinion in Infectious Diseases 30(1), 129–134 (2017).
- Ong C-T , Tsai C-F , Wong Y-S et al. Epidemiology of brain abscess in Taiwan: a 14-year population-based cohort study. PLOS ONE 12(5), e0176705 (2017).
- Calfee DP , Wispelwey B ( Eds). Brain abscess. Seminars in neurology. Thieme Medical Publishers, Inc., 333 Seventh Avenue (2000). Copyright© 2000 by New …
- Brouwer MC , Tunkel AR , McKhann GM et al. Brain Abscess. N. Engl. J. Med. 371(5), 447–456 (2014).
- Faraji RM , Samini F . Clinical features and outcome of 83 adult patients with brain abscess. Arch. Iranian Med. 10(3), 379–382 (2007).
- Mihai C , Jubelt B . Infectious Myelitis. Current Neurology and Neuroscience Reports 12(6), 633–641 (2012).
- Bigouette JP , Wilkinson AL , Tallis G et al. Progress Toward Polio Eradication—Worldwide, January 2019–June 2021. Morbidity and Mortality Weekly Report 70(34), 1129 (2021).
- Danaei G , Farzadfar F , Kelishadi R et al. Iran in transition. The Lancet 393(10184), 1984–2005 (2019).
- Shaghaghi M , Shahmahmoodi S , Abolhassani H et al. Vaccine-Derived Polioviruses and Children with Primary Immunodeficiency, Iran, 1995–2014. Emerg. Infect. Dis. 22(10), 1712–1719 (2016).
- Shoja ZO , Tabatabie H , Shahmahmoudi S et al. Comparison of cell culture with RT-PCR for enterovirus detection in stool specimens from patients with acute flaccid paralysis. J. Clin. Lab. Anal. 21(4), 232–236 (2007).
- Bajoghli M , Naficy AR , Vafai A et al. Paralytic poliomyelitis in Isfahan. The Journal of Tropical Pediatrics and Environmental Child Health 23(5), 236–238 (1977).
- Uprety P , Graf EH . Enterovirus infection and acute flaccid myelitis. Current Opinion in Virology 40, 55–60 (2020).
- Rahimi P , Tabatabaie H , Gouya MM et al. Direct identification of non-polio enteroviruses in residual paralysis cases by analysis of VP1 sequences. Journal of Clinical Virology 45(2), 139–141 (2009).
- Nejati A , Farahmand M , Tabatabaie H et al. Molecular typing of non-polio enteroviruses isolated from acute flaccid paralysis cases in Iran from 2010 to 2015. Virologica Sinica 32(3), 249–252 (2017).
- Nejati A , Zahraei SM , Mahmoudi S et al. Molecular characterization of non-polio enteroviruses isolated from children with acute flaccid paralysis in IRAN, 2015–2018. Virus Genes 56, 531–536 (2020).
- Yamauchi J , Araya N , Yagishita N et al. An update on human T-cell leukemia virus type I (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) focusing on clinical and laboratory biomarkers. Pharmacology & Therapeutics 218, 107669 (2021).
- Gessain A , Gessain A , Cassar O . Epidemiological Aspects and World Distribution of HTLV-1 Infection. Frontiers in Microbiology 3(388), 379–382 (2012).
- Parhizgari N , Gouya MM , Mostafavi E . Emerging and re-emerging infectious diseases in Iran. Iranian Journal of Microbiology 9(3), 122–142 (2017).
- Rafatpanah H , Hedayati-Moghaddam MR , Fathimoghadam F et al. High prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology survey. Journal of Clinical Virology: The Official Publication of the Pan American Society for Clinical Virology 52(3), 172–176 (2011).
- Araújo AQ , Andrade-Filho AS , Castro-Costa CM et al. HTLV-I-associated myelopathy/tropical spastic paraparesis in Brazil: a nationwide survey. HAM/TSP Brazilian Study Group. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology: Official Publication of the International Retrovirology Association 19(5), 536–541 (1998).
- Taylor GP , Tosswill JH , Matutes E et al. Prospective study of HTLV-I infection in an initially asymptomatic cohort. Journal of Acquired Immune Deficiency Syndromes 22(1), 92–100 (1999).
- Drevets DA , Leenen PJ , Greenfield RA . Invasion of the central nervous system by intracellular bacteria. Clin. Microbiol. Rev. 17(2), 323–347 (2004).
- Movahedi Z , Mahmoudi S , Mamishi S . Salmonella meningitis in a 16-month-old child with AIDS. The Turkish Journal of Pediatrics 55(2), 232–234 (2013).
- Khalili M , Naderi HR , Salehnia N et al. Detection of Coxiella burnetii in acute undifferentiated febrile illnesses (AUFIs) in Iran. Tropical Doctor 46(4), 221–224 (2016).
- Tabatabaie P , Syadati A . Bacillus anthracis as a cause of bacterial meningitis. The Pediatric Infectious Disease Journal 12(12), 1035–1037 (1993).
- Babamahmoodi F , Aghabarari F , Arjmand A et al. Three rare cases of anthrax arising from the same source. The Journal of Infection 53(4), e175–179 (2006).
- Khoddami M , Shirvani F , Esmaeili J et al. Two rare presentations of fatal anthrax: meningeal and intestinal. Archives of Iranian Medicine 13(5), 432–435 (2010).
- Chin JH . Neurotuberculosis: A Clinical Review. Semin. Neurol. 39(4), 456–461 (2019).
- Thakur K , Das M , Dooley KE et al.et al. ( Eds). The global neurological burden of tuberculosis. Seminars in neurology. Thieme Medical Publishers (2018).
- Kiani B , Raouf Rahmati A , Bergquist R et al. Spatio-temporal epidemiology of the tuberculosis incidence rate in Iran 2008 to 2018. BMC Public Health 21(1), 1093 (2021).
- Neshati H , Sheybani F , Naderi H et al. Diagnostic errors in tuberculous patients: a multicenter study from a developing country. Journal of Environmental and Public Health 2018 (2018).
- Leonard JM . Central nervous system tuberculosis. Tuberculosis and Nontuberculous Mycobacterial Infections 331–41 (2017).
- Tahernia AC . Tuberculous meningitis. Modern diagnosis, treatment and prognosis, as exemplified in 38 cases in southern Iran. Clin. Pediatr. 6(3), 173–177 (1967).
- Ahmadinejad Z , Ziaee V , Aghsaeifar M et al. The prognostic factors of tuberculous meningitis. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases 3(1), 1–7 (2003).
- Fallah F , Nasiri MJ , Pormohammad A . Bacillus Calmette-Guerin (BCG) vaccine in Iran. Journal of Clinical Tuberculosis and Other Mycobacterial Diseases 11, 22 (2018).
- Puvacić S , Dizdarević J , Santić Z et al. Protective effect of neonatal BCG vaccines against tuberculous meningitis. Bosnian Journal of Basic Medical Sciences 4(1), 46–49 (2004).
- Salmanzadeh S , Honarvar N , Goodarzi H et al. Chronic mycobacterial meningitis due to Mycobacterium chelonae: a case report. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases 27, 67–69 (2014).
- Sharifi G , Mousavinejad SA , Moradian K et al. Pineal Region Tuberculoma Caused by Mycobacterium bovis as a Complication of Bacille Calmette-Guérin Vaccine: Case Report and Review of the Literature. World Neurosurgery 133, 416–418 (2020).
- Dean AS , Crump L , Greter H et al. Global burden of human brucellosis: a systematic review of disease frequency. PLoS Neglected Tropical Diseases 6(10), e1865 (2012).
- Leylabadlo HE , Bialvaei AZ , Samadi Kafil H . Brucellosis in Iran: why not eradicated? Clin. Infect. Dis. 61(10), 1629–1630 (2015).
- Boschiroli M-L , Foulongne V , O’Callaghan D . Brucellosis: a worldwide zoonosis. Current Opinion in Microbiology 4(1), 58–64 (2001).
- Pappas G , Papadimitriou P , Akritidis N et al. The new global map of human brucellosis. The Lancet Infectious Diseases 6(2), 91–99 (2006).
- Naderi H , Sheybani F , Parsa A et al. Neurobrucellosis: report of 54 cases. Tropical Medicine and Health 50(1), 1–6 (2022).
- Piroozi B , Moradi G , Safari H et al. Incidence, mortality, and burden of human brucellosis and its geographical distribution in Iran during 2009–2015. Iranian Journal of Public Health 48(Suppl. 1), 20–27 (2019).
- Turgut M , Haddad FS , De Divitiis O . Neurobrucellosis: Clinical, diagnostic and therapeutic features. Springer (2015).
- Buzgan T , Karahocagil MK , Irmak H et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. International Journal of Infectious Diseases 14(6), e469–e478 (2010).
- Gul HC , Erdem H , Bek S . Overview of neurobrucellosis: a pooled analysis of 187 cases. International Journal of Infectious Diseases 13(6), e339–e343 (2009).
- Mandell G , Raphael D , John B . Mandell, Douglas, and Bennett’s principles and practice of infectious diseases (9th ed.). Elsevier (2019).
- Hatami H , Hatami M , Soori H et al. Epidemiological, clinical, and laboratory features of brucellar meningitis. Archives of Iranian Medicine 13(6), 486–491 (2010).
- Asadipooya K , Dehghanian A , Omrani GH et al. Short-course treatment in neurobrucellosis: a study in Iran. Neurology India 59(1), 101–103 (2011).
- Ranjbar M , Rezaiee AA , Hashemi SH et al. Neurobrucellosis: report of a rare disease in 20 Iranian patients referred to a tertiary hospital. Eastern Mediterranean Health Journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit 15(1), 143–148 (2009).
- Ayatollahi J . Epidemiological, clinical, diagnostic and therapeutic survey of 686 cases of brucellosis. Annals of Saudi Medicine 24(5), 398–399 (2004).
- Roushan MH , Mohrez M , Gangi SS et al. Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, Northern Iran. Epidemiology & Infection 132(6), 1109–1114 (2004).
- Karsen H , Tekin Koruk S , Duygu F et al. Review of 17 cases of neurobrucellosis: clinical manifestations, diagnosis, and management. Archives of Iranian Medicine 15(8), 491–494 (2012).
- Haji-Abdolbagi M , Rasooli-Nejad M , Jafari S et al. Clinical and laboratory findings in neurobrucellosis: review of 31 cases. Archives of Iranian Medicine 11(1), 21–25 (2008).
- Naderi HR . Brucellar Psychosis. Neurobrucellosis. Springer, 69–79 (2016).
- Bidaki R , Yassini S , Maymand M et al. Acute psychosis due to brucellosis in a rural Iran: a report of two cases. International Journal of Infection and Microbiology 2(1), 29–31 (2013).
- Tonekaboni SH , Karimi A , Armin S et al. Neurobrucellosis: a partially treatable cause of vision loss. Pediatr. Neurol. 40(5), 401–403 (2009).
- Faraji F , Didgar F , Talaie-Zanjani A et al. Uncontrolled seizures resulting from cerebral venous sinus thrombosis complicating neurobrucellosis. Journal of Neurosciences in Rural Practice 4(03), 313–316 (2013).
- Montazeri M , Sadeghi K , Khalili H et al. Fever and psychosis as an early presentation of Brucella-associated meningoencephalitis: a case report. Medical Principles and Practice: international Journal of the Kuwait University, Health Science Centre 22(5), 506–509 (2013).
- Sheybani F , Sarvghad MR , Bojdi A et al. Brief Report: Brucellar Psychosis. Archives of Iranian Medicine 15(11), 723–725 (2012).
- Dadar M , Alamian S , Behrozikhah AM et al.et al. ( Eds). Molecular identification of Brucella species and biovars associated with animal and human infection in Iran. Veterinary research forum. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran (2019).
- Koopmans MM , Bijlsma MW , Brouwer MC et al. Listeria monocytogenes meningitis in the Netherlands, 1985–2014: a nationwide surveillance study. The Journal of Infection 75(1), 12–19 (2017).
- Zahedi Bialvaei A , Sheikhalizadeh V , Mojtahedi A et al. Epidemiological burden of Listeria monocytogenes in Iran. Iran J. Basic Med. Sci. 21(8), 770–780 (2018).
- Bazooyar B . Rhombencephalitis by Listeria Monocytogenes in Two Diabetic Patients. Archives of Iranian Medicine 18(9), 613–615 (2015).
- Spelman D . Microbiology, epidemiology, and pathogenesis of nocardiosis (2021). https://www.uptodate.com/contents/microbiology-epidemiology-and-pathogenesis-of-nocardiosis?search=NOCARDIA&source=search_result&selectedTitle=2∼94&usage_type=default&display_rank=2#H2
- Hashemzadeh M , Dezfuli AAZ , Khosravi AD et al. Genotypic and phenotypic prevalence of Nocardia species in Iran: first systematic review and meta-analysis of data accumulated over years 1992–2021. PLOS ONE 16(7), e0254840 (2021).
- Marra CM . Neurosyphilis (2022). https://www.uptodate.com/contents/neurosyphilis?search=neurosyphilis&source=search_result&selectedTitle=1∼71&usage_type=default&display_rank=1
- Marra CM . Neurosyphilis (2022). https://www.uptodate.com/contents/neurosyphilis?search=neurosyphilis&source=search_result&selectedTitle=1∼70&usage_type=default&display_rank=1
- Jahanbakhsh F , Bagheri Amiri F , Sedaghat A et al. Prevalence of HAV Ab, HEV (IgG), HSV2 IgG, and Syphilis Among Sheltered Homeless Adults in Tehran, 2012. Int. J. Health Policy Manag. 7(3), 225–230 (2018).
- Ghorashi Z . Sexually transmitted infections in Iran: a literature review. Journal of Occupational Health and Epidemiology 4, 260–265 (2015).
- Janghorban R . An overview on sexually transmitted infections in Iran. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 5, 585–595 (2016).
- Khodabandeh M , Borhani K , Eshaghi H et al. Neurosyphilis with Hydrocephalus: A Case Report. Case Reports in Clinical Practice 2(4), 98–102 (2017).
- Cadavid D , Barbour AG . Neuroborreliosis during relapsing fever: review of the clinical manifestations, pathology, and treatment of infections in humans and experimental animals. Clin. Infect. Dis. 26(1), 151–164 (1998).
- Assous M , Wilamowski A . Relapsing fever borreliosis in Eurasia—forgotten, but certainly not gone! Clinical Microbiology and Infection 15(5), 407–414 (2009).
- Assmar M , Soleimani M , Oreiz F et al. Purification of periplasmic flagellar antigen from Borrelia microtti. Scandinavian Journal of Infectious Diseases 34(4), 267–272 (2002).
- Masoumi Asl H , Goya MM , Vatandoost H et al. The epidemiology of tick-borne relapsing fever in Iran during 1997–2006. Travel Medicine and Infectious Disease 7(3), 160–164 (2009).
- Majid-Pour A . A case of Borrelia meningitis. Arch of Iranian Med. 6(3), 222–223 (2003).
- Masuzawa T . Terrestrial distribution of the Lyme borreliosis agent Borrelia burgdorferi sensu lato in East Asia. Japanese Journal of Infectious Diseases 57(6), 229–235 (2004).
- Koedel U , Fingerle V , Pfister H-W . Lyme neuroborreliosis—epidemiology, diagnosis and management. Nature Reviews Neurology 11(8), 446–456 (2015).
- Adabi M , Rahmani Firouzjaei A , Ghasemi M . Report of A Case of Lyme Disease in Mazandaran. Iranian Journal of Dermatology 8(Suppl. 1), (2004).
- Siadati PTA . A case of Lyme disease (Lyme Borreliosis). Acta Medica Iranica 4(3), 222–4 (2006).
- Chams-Davatchi C . The First Endemic Case of Lyme Borreliosis in Iran. Medical Journal of The Islamic Republic of Iran (MJIRI) 11(3), 237–239 (1997).
- Haake DA , Levett PN . Leptospirosis in humans. Leptospira and Leptospirosis 387, 65–97 (2015).
- Day N . Leptospirosis: Epidemiology, microbiology, clinical manifestations, and diagnosis (2022). https://www.uptodate.com/contents/leptospirosis-epidemiology-microbiology-clinical-manifestations-and-diagnosis?search=leptospirosis&source=search_result&selectedTitle=1∼84&usage_type=default&display_rank=1#H6605847
- Moradi G , Asl HM , Bahmani N et al. Epidemiology incidence and geographical distribution of leptospirosis using GIS and its incidence prediction in Iran in 2021. Medical Journal of the Islamic Republic of Iran 35, 109 (2021).
- Khalili M , Sakhaee E , Bagheri Amiri F et al. Serological evidence of leptospirosis in Iran; A systematic review and meta-analysis. Microb. Pathog. 138, 103833 (2020).
- Kheir A , Nabavi M , Tahaminia M . Rate of leptospiral meningitis and meningoencephalitis in Tehran. Iranian Journal of Clinical Infectious Diseases 4(4), 233–236 (2009).
- Babamahmoodi F , Babamhmoodi A . Recovery from Intracranial Hemorrhage Due to Leptospirosis. Case Reports in Medicine 2011, 504308 (2011).
- Góralska K , Blaszkowska J , Dzikowiec M . Neuroinfections caused by fungi. Infection 46, 443–459 (2018).
- Williamson PR , Jarvis JN , Panackal AA et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nature Reviews Neurology 13(1), 13–24 (2017).
- Cox GM , Perfect JR . Microbiology and epidemiology of Cryptococcus neoformans infection (2022). https://www.uptodate.com/contents/microbiology-and-epidemiology-of-cryptococcus-neoformans-infection?search=cryptococcosis&source=search_result&selectedTitle=2∼150&usage_type=default&display_rank=2
- Pappas PG , Perfect JR , Cloud GA et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America 33(5), 690–699 (2001).
- Bandalizadeh Z , Hosseini SA , Moosazadeh M et al. Cryptococcus and cryptococcosis in Iran during 1969–2019: a systematic review and meta-analysis. Journal de Mycologie Medicale 30(1), 100917 (2019).
- Ghasemian R , Najafi N , Shokohi T . Cryptococcal meningitis relapse in an immunocompetent patient. Archives of Clinical Infectious Diseases 6(1), 51–55 (2011).
- Razin B , Shoaei S-D , Family A et al. PP-204 Mycobacterium tuberculosis and Cryptococcus neoformans coinfection meningitis in a young immunocompetent woman. International Journal of Infectious Diseases 15, S101 (2011).
- Nabaei G , Afhami S . Disseminated cryptococcosis and active pulmonary tuberculosis co-infection in an otherwise healthy adult. Iranian Journal of Neurology 14(3), 174 (2015).
- Badali H , Alian S , Fakhim H et al. Cryptococcal meningitis due to Cryptococcus neoformans genotype AFLP 1/VNI in Iran: a review of the literature. Mycoses 58(12), 689–693 (2015).
- Nakhayi AR , Eftekhaari TE , Montazerghaem H et al. A 28years old woman with severe headache and few episodes of vomiting: a case report. American Journal of Infectious Diseases 6(4), 107 (2010).
- Sasan MS , Donyadideh N , Alborzi A . A case report of cryptococcal meningitis. Medical Journal of Mashhad University of Medical Sciences 55(3), 190–194 (2012).
- Siroos B , Ahmadinejad Z , Tabaeizadeh M et al. Rare association of severe cryptococcal and tuberculosis in central nervous system in a case of sarcoidosis. Medical Journal of the Islamic Republic of Iran 28(1), 22 (2014).
- Khodadoust AA , Payne JW . Cryptococcal (torular) retinitis. A clinicopathologic case report. Am. J. Ophthalmol. 67(5), 745–750 (1969).
- Haghighi S , Ahadi MS , Moghadasi AN . Cryptococcal meningitis in a human immunodeficiency virus-negative patient with rheumatoid arthritis. Iranian Journal of Neurology 15(2), 106 (2016).
- Bassiri Jahromi S , Khaksar AA . Deep-Seated Fungal Infections in Immunocompromised Patients in Iran before and after Treatments. Iranian Journal of Allergy, Asthma and Immunology 4(1), 27–32 (2005).
- Aghazadeh K , Nadji SA , Shokouhi S et al. Concurrent presence of cryptococcal meningitis and neoplastic meningitis in a recipient of hematopoietic stem cell transplantation: a case report. Crescent Journal of Medical and Biological Sciences 3(2), 45–50 (2016).
- Amanati A , Lotfi M , Masoudi MS et al. Cerebral and pulmonary aspergillosis, treatment and diagnostic challenges of mixed breakthrough invasive fungal infections: case report study. BMC Infectious Diseases 20(1), 1–7 (2020).
- Mamishi S , Parvaneh N , Salavati A et al. Invasive aspergillosis in chronic granulomatous disease: report of 7 cases. Eur. J. Pediatr. 166(1), 83–84 (2007).
- Azarpira N , Esfandiari M , Bagheri MH et al. Cerebral aspergillosis presenting as a mass lesion. Brazilian Journal of Infectious Diseases 12(4), 349–351 (2008).
- Bassiri-Jahromi S . A retrospective study of the risk factors for invasive Aspergillosis in Iran. Virol Mycol. 2(1), 2161–0517 (2013).
- Safavi M , Mehdizadeh M , Habibi Z et al. Cerebral Aspergillosis. Archives of Iranian Medicine 22(8), 476–477 (2019).
- Petramfar P , Yousefian M , Ashraf MH et al. Meningoencephalitis with Aspergillus and mycobacterium tuberculosis in a renal transplant recipient. Experimental and clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 9(1), 68–71 (2011).
- Badiee P , Alborzi A , Malekhosseini SA et al. Determining the incidence of aspergillosis after liver transplant. Experimental and Clinical Transplantation: Official Journal of the Middle East Society for Organ Transplantation 8(3), 220–223 (2010).
- Amanati A , Lotfi M , Masoudi MS et al. Cerebral and pulmonary aspergillosis, treatment and diagnostic challenges of mixed breakthrough invasive fungal infections: case report study. BMC Infectious Diseases 20(1), 535 (2020).
- Góralska K , Blaszkowska J , Dzikowiec M . Neuroinfections caused by fungi. Infection 46(4), 443–459 (2018).
- Cox G , Kauffman C , Bond S . Mucormycosis (zygomycosis) (2021). https://www.uptodate.com/contents/mucormycosis-zygomycosis.
- Nagy-Agren SE , Chu P , Smith GJ et al. Zygomycosis (mucormycosis) and HIV infection: report of three cases and review. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology: Official Publication of the International Retrovirology Association 10(4), 441–449 (1995).
- Vaezi A , Moazeni M , Rahimi MT et al. Mucormycosis in Iran: a systematic review. Mycoses 59(7), 402–415 (2016).
- Dolatabadi S , Ahmadi B , Rezaei-Matehkolaei A et al. Mucormycosis in Iran: a six-year retrospective experience. Journal de Mycologie Medicale 28(2), 269–273 (2018).
- Einollahi B , Lessan-Pezeshki M , Aslani J et al. Two decades of experience in mucormycosis after kidney transplantation. Annals of Transplantation 16(3), 44–48 (2011).
- Kashkouli MB , Abdolalizadeh P , Oghazian M et al. Outcomes and factors affecting them in patients with rhino-orbito-cerebral mucormycosis. The British Journal of Ophthalmology 103(10), 1460–1465 (2019).
- Nezafati S , Kazemi A , Asgari K et al. Rhinocerebral mucormycosis, risk factors and the type of oral manifestations in patients referred to a University Hospital in Tabriz, Iran 2007–2017. Mycoses 61(10), 764–769 (2018).
- Samarei R , Gharebaghi N , Zayer S . Evaluation of 30 cases of mucormycosis at a university hospital in Iran. Mycoses 60(7), 426–432 (2017).
- Sanavi S , Afshar R , Afshin-Majd S . Rhino-orbitocerebral mucormycosis in a patient with idiopathic crescentic glomerulonephritis. Saudi Journal of Kidney Diseases and Transplantation: An Official Publication of the Saudi Center for Organ Transplantation, Saudi Arabia 24(4), 768–772 (2013).
- Cox GM , Kauffman CA . Central nervous system infections due to dematiaceous fungi (cerebral phaeohyphomycosis) (2021). https://www.uptodate.com/contents/central-nervous-system-infections-due-to-dematiaceous-fungi-cerebral-phaeohyphomycosis?search=Central%20nervous%20system%20infections%20due%20to%20dematiaceous%20fungi%20(cerebral%20phaeohyphomycosis)&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Revankar SG , Sutton DA , Rinaldi MG . Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 38(2), 206–216 (2004).
- Geramishoar M , Zomorodian K , Zaini F et al. First case of cerebral phaeohyphomycosis caused by Nattrassia mangiferae in Iran. Japanese Journal of Infectious Diseases 57(6), 285–286 (2004).
- Badali H , Chander J , Gupta A et al. Fatal cerebral phaeohyphomycosis in an immunocompetent individual due to Thielavia subthermophila. J. Clin. Microbiol. 49(6), 2336–2341 (2011).
- Didehdar M , Gokanian A , Sofian M et al. First fatal cerebral phaeohyphomycosis due to Rhinocladiella mackenziei in Iran, based on ITS rDNA. Journal de Mycologie Medicale 25(1), 81–86 (2015).
- Saberi H , Kashfi A , Hamidi S et al. Cerebral phaeohyphomycosis masquerading as a parafalcian mass: case report. Surg. Neurol. 60(4), 354–9; discussion 9 (2003).
- Bassiri Jahromi S , Khaksar AA . Deep-seated fungal infections in immunocompromised patients in iran. Iranian Journal of Allergy, Asthma, and Immunology 4(1), 27–32 (2005).
- Basiri K , Meidani M , Rezaie F et al. A rare case of Trichosporon brain abscess, successfully treated with surgical excision and antifungal agents. Neurologia i Neurochirurgia Polska 46(1), 92–95 (2012).
- Padayachy LC , Dattatraya M . Hydatid disease (Echinococcus) of the central nervous system. Childs Nerv. Syst. 34(10), 1967–1971 (2018).
- Carpio A , Romo ML , Parkhouse R et al. Parasitic diseases of the central nervous system: lessons for clinicians and policy makers. Expert Review of Neurotherapeutics 16(4), 401–414 (2016).
- Carpio A . Neurocysticercosis: an update. The Lancet Infectious Diseases 2(12), 751–762 (2002).
- Bizhani N , Hashemi Hafshejani S , Mohammadi N et al. Human Cysticercosis in Asia: A Systematic Review and Meta-Analysis. Iranian Journal of Public Health 49(10), 1839–1847 (2020).
- Farahani ZK , Moradi A , Sedighi A et al. Neurocysticercosis in Iran: An Unexpected Case and Literature Review. Neuroscience and Medicine 04(04), 5 (2013).
- Moazeni M , Khamesipour F , Anyona DN et al. Epidemiology of taeniosis, cysticercosis and trichinellosis in Iran: a systematic review. Zoonoses and Public Health 66(1), 140–154 (2019).
- Moro P , Weller P , Baron EL . Epidemiology and control of echinococcosis (2020). https://www.uptodate.com/contents/epidemiology-and-control-of-echinococcosis?search=Clinical%20manifestations%20and%20diagnosis%20of%20cystic%20and%20alveolar%20echinococcosis&source=search_result&selectedTitle=2∼150&usage_type=default&display_rank=2
- Mahmoudi S , Mamishi S , Banar M et al. Epidemiology of echinococcosis in Iran: a systematic review and meta-analysis. BMC Infectious Diseases 19(1), 929 (2019).
- Fasihi Harandi M , Budke CM , Rostami S . The monetary burden of cystic echinococcosis in Iran. PLoS Neglected Tropical Diseases 6(11), e1915 (2012).
- Nourjah N , Sahba G , Baniardalani M et al. Study of 4850 operated hydatidosis cases in Iran. The Southeast Asian Journal of Tropical Medicine and Public Health 35(1), 218–222 (2004).
- Varo R , Crowley VM , Sitoe A et al. Adjunctive therapy for severe malaria: a review and critical appraisal. Malar J. 17(1), 47 (2018).
- Postels DG , Birbeck GL . Cerebral malaria. Handbook of Clinical Neurology 114, 91–102 (2013).
- Vatandoost H , Raeisi A , Saghafipour A et al. Malaria situation in Iran: 2002–2017. Malar J. 18(1), 200 (2019).
- Vatandoost H , Raeisi A , Saghafipour A et al. Malaria situation in Iran: 2002–2017. Malar J. 18(1), 200 (2019).
- Azizi H , Davtalab-Esmaeili E , Farahbakhsh M et al. Malaria situation in a clear area of Iran: an approach for the better understanding of the health service providers’ readiness and challenges for malaria elimination in clear areas. Malaria Journal 19(1), 114 (2020).
- Ziaee M , Abedi F . Severe falciparum malaria in iran: a very rare case from an endemic region. Jundishapur Journal of Microbiology 7(1), e8752 (2014).
- Solimani GR , Keshavarz K . Cerebral Malaria in Children: A Case Report. Iranian Journal of Pediatrics 14(2), 163–166 (2004).
- Elsheikha HM , Marra CM , Zhu X-Q . Epidemiology, pathophysiology, diagnosis, and management of cerebral toxoplasmosis. Clin. Microbiol. Rev. 34(1), e00115–00119 (2020).
- Daryani A , Sarvi S , Aarabi M et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Tropica 137, 185–194 (2014).
- Ahmadpour E , Daryani A , Sharif M et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. The Journal of Infection in Developing Countries 8(12), 1503–1510 (2014).
- Sharifi-Mood B , Alavi-Naini R , Salehi M et al. Spectrum of clinical disease in a series of hospitalized HIV-infected patients from southeast of Iran. Saudi Medical Journal 27(9), 1362–1366 (2006).
- Mohraz M , Jozani ZB , Behtaj M et al. Neurological manifestations in HIV positive patients in Tehran, Iran. Asian Pacific Journal of Tropical Disease 4, S481–S485 (2014).
- Abbasian L , Hashemi H , Zali A et al. Incidence and Properties of Neurologic Disorders Recovered from Iranian Patients with HIV Infection: A Case Series. Archives of Neuroscience 6(4), e81981 (2019).
- Boughattas S . Toxoplasma infection and milk consumption: meta-analysis of assumptions and evidences. Crit. Rev. Food Sci. Nutr. 57(13), 2924–2933 (2017).
- Abbasi Fard S , Khajeh A , Khosravi A et al. Fulminant and Diffuse Cerebral Toxoplasmosis as the First Manifestation of HIV Infection: A Case Presentation and Review of the Literature. Am. J. Case Rep. 21, e919624–e (2020).
- Abolghasemi H , Shahverdi E , Jafari R et al. Central Nervous System Toxoplasmosis in Relapsed Hodgkin’s Lymphoma: A Case Report. Iran J. Cancer Prev. 9(4), e5810 (2016).
- Nguyen L . Amebic Meningoencephalitis. WebMD (2021). https://emedicine.medscape.com/article/996227-overview
- Nguyen L , Perloff S . Amebic Meningoencephalitis. Medscape Publishers’ circle (2016). https://emedicine.medscape.com/article/996227-overview#a4
- Niyyati M , Karamati SA , Lorenzo Morales J et al. Isolation of Balamuthia mandrillaris from soil samples in North-Western Iran. Parasitol. Res. 115(2), 541–545 (2016).
- Badirzadeh A , Niyyati M , Babaei Z et al. Isolation of free-living amoebae from sarein hot springs in ardebil province, iran. Iranian Journal of Parasitology 6(2), 1 (2011).
- Binesh F , Karimi M , Navabii H . Unexpected postmortem diagnosis of Acanthamoeba meningoencephalitis in an immunocompetent child. Case Reports 2011, bcr0320113954 (2011).
- Movahedi Z , Shokrollahi MR , Aghaali M et al. Primary amoebic meningoencephalitis in an Iranian infant. Case Reports in Medicine 2012, (2012).
- Binesh F , Karimi M , Navabii H . Unexpected postmortem diagnosis of acanthamoeba meningoencephalitis in an immunocompetent child. BMJ Case Reports 2011, (2011).
- Movahedi Z , Shokrollahi MR , Aghaali M et al. Primary amoebic meningoencephalitis in an Iranian infant. Case Rep. Med. 2012, 782854 (2012).
- Abbaszadeh Afshar MJ , Zahabiun F , Heydarian P et al. A systematic review and meta-analysis of toxocariasis in Iran: is it time to take it seriously? Acta Parasitologica 65(3), 569–584 (2020).
- World Health Organization . Schistosomiasis. World Health Organization (2022). www.who.int/news-room/fact-sheets/detail/schistosomiasis
- Haghighi L , Akbaribazm M , Arab-Mazar Z et al. Vesical schistosomiasis and squamous cell carcinoma associated with schistosoma haematobium: a re-emerging neglected tropical disease in Tehran, Iran. Urology Case Reports 30, 101140 (2020).
- Khademvatan S , Salmanzadeh S , Foroutan-Rad M et al. Elimination of urogenital schistosomiasis in Iran: past history and the current situation. Parasitology 143(11), 1390–1396 (2016).
- Chitsulo L , Engels D , Montresor A et al. The global status of schistosomiasis and its control. Acta Tropica 77(1), 41–51 (2000).
- Alavi SM , Salmanzadeh S . Schistosomiasis in Iran, From the Past Till Elimination 3(3), e60152 (2016).
- Alavi SM , Salmanzadeh S . Schistosomiasis in Iran, from the past till elimination. Int. J. Infect. 3(3), e36075 (2016).
- Rose MF , Zimmerman EE , Hsu L et al. Atypical presentation of cerebral schistosomiasis four years after exposure to Schistosoma mansoni. Epilepsy & Behavior Case Reports 2, 80–85 (2014).
- Chanda K . An overview on the therapeutics of neglected infectious diseases—Leishmaniasis and Chagas diseases. Frontiers in Chemistry 9, 622286 (2021).
- Petersen CA , Greenlee MHW . Neurologic manifestations of Leishmania spp. Infection. Journal of Neuroparasitology 2, (2011).
- Llanos-Cuentas A , Valencia BM , Petersen CA . Chapter 13 - Neurological manifestations of human leishmaniasis. In: Handbook of Clinical Neurology. Garcia HH , Tanowitz HB , Del Brutto OH ( Eds). Elsevier, 114: 193–198 (2013).
- Mohebali M . Visceral leishmaniasis in Iran: review of the epidemiological and clinical features. Iranian Journal of Parasitology 8(3), 348 (2013).
- Sedaghattalab M , Azizi A . Brain Parenchyma (pons) Involvement by Visceral Leishmaniasis: A Case Report. Iranian Journal of Parasitology 13(1), 145–148 (2018).
- Moncayo A , Guhl F , Stein C . The Global Burden of Chagas’ Disease in the Year 2000. GBD 2000 Working Paper. World Health Organization, Geneva (2002).
- Rassi A , de Rezende JM . American trypanosomiasis (Chagas disease). Infectious Disease Clinics 26(2), 275–291 (2012).
- Córdova E , Maiolo E , Corti M et al. Neurological manifestations of Chagas’ disease. Neurol. Res. 32(3), 238–244 (2010).
- Prusiner SB . Neurodegenerative Diseases and Prions. N. Engl. J. Med. 344(20), 1516–1526 (2001).
- Appleby BS , Cohen ML . Creutzfeldt-Jakob disease (2020). https://www.uptodate.com/contents/creutzfeldt-jakob-disease?search=prion%20disease&topicRef=5074&source=see_link#H29
- Hermann P , Appleby B , Brandel J-P et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. The Lancet Neurology 20(3), 235–246 (2021).
- Appleby BS , Cohen ML . Creutzfeldt-Jakob disease (2020). https://www.uptodate.com/contents/creutzfeldt-jakob-disease?search=Creutzfeldt-Jakob%20disease&source=search_result&selectedTitle=1∼93&usage_type=default&display_rank=1
- Ritchie DL , Peden AH , Barria MA . Variant CJD: reflections a Quarter of a Century on. Pathogens (Basel, Switzerland) 10(11), 1413 (2021).
- Watson N , Brandel JP , Green A et al. The importance of ongoing international surveillance for Creutzfeldt-Jakob disease. Nature Reviews Neurology 17(6), 362–379 (2021).
- Coulthart MB , Geschwind MD , Qureshi S et al. A case cluster of variant Creutzfeldt-Jakob disease linked to the Kingdom of Saudi Arabia. Brain: A Journal of Neurology 139(Pt 10), 2609–2616 (2016).
- Sarraf P , Ghajarzadeh M , Salarian B . Creutzfeldt-Jacob disease: a case report. Acta Medica Iranica 52(6), 488–489 (2014).
- Masullo C , Brown PW , Macchi G . Creutzfeldt-Jakob disease in an Iranian: the first clinico-pathologically described case. Clin. Neuropathol. 15(1), 26–29 (1996).
- Nikanfar M , Farhoudi M , Halimi M et al. Creutzfeldt-Jakob disease presenting with confusion and visual disturbance. Archives of Iranian Medicine 10(3), 397–400 (2007).
- Sheybani F , van de Beek D , Brouwer MC . Suspected Central Nervous System Infections in HIV-Infected Adults. Front Neurol. 12, 741884 (2021).
- Boissé L , Gill MJ , Power C . HIV Infection of the Central Nervous System: Clinical Features and Neuropathogenesis. Neurol. Clin. 26(3), 799–819 (2008).
- Boufassa F , Bachmeyer C , Carré N et al. Influence of neurologic manifestations of primary human immunodeficiency virus infection on disease progression. SEROCO Study Group. The Journal of Infectious Diseases 171(5), 1190–1195 (1995).
- UNAIDS . Islamic Republic of Iran. UNAIDS (2020). www.unaids.org/en/regionscountries/countries/islamicrepublicofiran
- Wang Y , Liu M , Lu Q et al. Global prevalence and burden of HIV-associated neurocognitive disorder. A Meta-Analysis 95(19), e2610–e2621 (2020).
- Taquet M , Geddes JR , Husain M et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet Psychiatry 8(5), 416–427 (2021).
- Brueggemann AB , Jansen van Rensburg MJ , Shaw D et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. The Lancet Digital Health 3(6), e360–e370 (2021).
- de Gier B , Marchal N , de Beer-Schuurman I et al. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Euro Surveillance: Bulletin Europeen sur les maladies transmissibles=European Communicable Disease Bulletin 28(1), 2200941 (2023).
- Nabizadeh F , Balabandian M , Sodeifian F et al. Autoimmune encephalitis associated with COVID-19: a systematic review. Multiple Sclerosis and Related Disorders 62, 103795 (2022).
- Saade A , Moratelli G , Azoulay E et al. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infectious Diseases Now. 51(8), 676–679 (2021).
- Aslan C , Nikfarjam S , Asadzadeh M et al. Neurological manifestations of COVID-19: with emphasis on Iranian patients. J. Neurovirol. 27, 217–227 (2021).
- Nejad JH , Allahyari F , Hosseinzadeh R et al. Neurological symptoms of COVID-19 infection; a cross-sectional study on hospitalized COVID-19 patients in Iran. Clin. Neurol. Neurosurg. 210, 106985 (2021).
- Haddad M , Sheybani F , Olfati N et al. Central nervous system reactivation of herpesviridae family in patients with COVID-19. J. Neurovirol. 29(2), 211–217 (2023).
- Hosseini MJ , Halaji M , Nejad JH et al. Central nervous system vasculopathy associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a novel case report from Iran. J. Neurovirol. 27, 507–509 (2021).
- Communicable disease surveillance systems. World Health Organization (2023). www.emro.who.int/fr/health-topics/health-information-systems/communicable-disease-surveillance-systems.html
- Dikid T , Jain S , Sharma A et al. Emerging & re-emerging infections in India: an overview. The Indian Journal of Medical Research 138(1), 19 (2013).
- HabibiSaravi R , Khankeh H , Azar A et al. Research Paper: Communicable Diseases Surveillance System in Iran: Strengths and Weaknesses 30 Years Following its Implementation. Health in Emergencies and Disasters Quarterly 5(1), 25–36 (2019).
- Turnberg W , Daniell W , Duchin J . Notifiable infectious disease reporting awareness among physicians and registered nurses in primary care and emergency department settings. Am. J. Infect. Control 38(5), 410–412 (2010).
- Kolahi A-A , Bakhshaei P , Ahmadnia H et al. The Viewpoints of General Practitioners Owning a Private office in North and East of Tehran about Barriers and Problems of Reporting of Communicable Diseases in 2011. Iranian Journal of Infectious Diseases and Tropical Medicine 18(62), (2013).
- Dehcheshmeh NF , Arab M , Foroushani AR et al. Survey of communicable diseases surveillance system in hospitals of Iran: a qualitative approach. Global Journal of Health Science 8(9), 44 (2016).
- Mouseli A , Amiresmaili M , Barouni M et al. The Challenges and Barriers to the Integration of Laboratory Services in Iran from Laboratory Experts’ Point of View. Iranian Journal of Public Health 47(6), 884 (2018).