ABSTRACT
Background
Much of the research into the use of drama in science education has been qualitative, with its primary focus being on its affective value amongst students aged 6–14 years, with a smaller fraction on effectiveness in terms of academic achievement and understanding of chemistry amongst older school students (16–18 years).
Purpose
This article reports on a study that compared the effectiveness of drama, as a pedagogy, with a didactic practice-examinations-questions-based approach on students’ understanding of chemistry organic reaction mechanisms as measured by examination question scores.
Sample
Data were collected in seven 11–18 schools and a Further Education (FE) college in England, with a total of 236 students, aged 16–18 years, studying Advanced (A) Level chemistry. Each institution had two different classes studying chemistry concurrently.
Design and methods
The research was a quasi-experimental intervention with one class of A Level chemistry students in each institution being taught an aspect of organic reaction mechanisms using drama and the second, non-drama, class being taught the same material using practice examination questions. Post-intervention, all students completed previously unseen A Level examination questions and, in one phase of the study, a diagnostic question designed to probe deeper understanding. The responses were subject to statistical analysis.
Results
In all cases there were no statistically significant differences between the test scores of the two groups for the answers to the examination questions. However, answers to a diagnostic question, probing deep level understanding, showed a statistically significant difference between the two groups in favour of the drama group.
Conclusions
Whilst drama is at least as effective as the use of practice examination questions in the teaching and learning of organic reaction mechanisms it was statistically significantly more effective in terms of the development of deeper level conceptual understanding.
Introduction
Drama in science education
Drama is an activity making use of a written script as the basis for individuals to tell stories and explore meaning via non-verbal actions (performance) and dialogue (Marsella, Johnson, and LaBore Citation2000). Baskerville, McGregor, and Bonsall (Citation2023) draw attention to categories of learning that take place by using drama; ‘learning from’ (p19) by watching a drama, ‘learning in’ (p19) by participating in drama and ‘learning through’ (p19) by writing a drama production. This study focuses on the latter two forms of learning. Research into the use of drama in science education has tended to focus on the age group 6–14 years, unlike the present study that used simulation-role-play (SRP), a subset of drama, as a classroom pedagogy to assist learning of an aspect of chemistry with students aged 16–18 years. There are a number of studies relating to the use of drama for exploring historical and societal aspects of the science curriculum, while other studies correspond to the teaching and learning of scientific content. Whilst the former provide wider context, the latter are more relevant to this study. An interrogation of this latter segment of the literature reveals a number of studies making claims regarding the learning of science content through the use of drama as a classroom pedagogy; some studies providing anecdotal reports of improvements in scientific knowledge e.g. Dawson et al., (Citation2009). Hendrix, Eick and Shannon (Citation2012) showed students aged 9–11 years, using drama, performed statistically significantly better than the control group in post-intervention tests relating to sound and solar energy. Similarly, 92% of children aged 5–7 years in McGregor’s (Citation2012) study reported the use of drama, in their context-based lessons, helped them to understand difficult ideas in science.
Working with students aged 13 years, Abed (Citation2016), using guided SRP, concluded that the use of drama to teach particle behaviour led to a statistically significant increase in scientific knowledge for the drama group compared to the non-drama group. Students and teachers reported that drama helped in the teaching of difficult topics in Arieli’s (Citation2007) study with students aged 11–12 years learning about mixtures and solutions. Metcalfe et al. (Citation1984), in a study of students aged 10–11 years, using drama to learn change of state reported no statistically significant differences for factual recall, but that the drama group performed statistically significantly better than the control group in explanation and interpretation questions.
In order to investigate how drama-based science activities operate in the classroom, Ødergaard (Citation2003) considered two continuums: one ranging between spontaneous to structured, the other from student-directed to teacher-directed and these ideas were incorporated into the present study ().
Figure 1. Forms of organisation in ‘dramatic scene’ activities (Ødergaard, Citation2003, p.79).
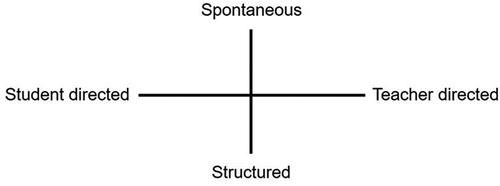
Ødergaard (Citation2003) argued that experiential drama is used to allow a student to become immersed in a situation and that, irrespective of the mode of drama, students are able to rework and reconstruct (or construct) meaning from these experiences.
Drama-based activities in science education can be categorised. McGregor and Anderson (Citation2023) exemplify the efficacy of different types of drama, dependent on the science learning required. These aspects of science education include conceptual understanding, the nature of science, and socio scientific issues. Literature pertaining to conceptual understanding and the use of drama often cites the use of role play, a sub set of drama, as the preferred choice. In role-play the student assumes the part of inanimate objects to support symbolic role-play in order to introduce scientific concepts (Metcalfe et al. Citation1984). Students use their bodies to represent the activity of these objects, e.g. particles in a chemical reaction. This idea of bodily movement playing a part in creating meaning is referred to as embodied learning; Alibali and Nathan (Citation2012) argue that body shape and movement serve to inform and apprise mental processes.
Aubusson et al. (Citation1997) refer to this type of role-play as simulation-role-play SRP, with Dorion (Citation2009) noting that in the existing literature this type of role-play is variously referred to by any of the following terms: ‘drama models, role play simulations, drama machines, analogy drama and metaphorical role play’ (p.2251). The use of SRP has been adopted in cases such as Abed (Citation2016); Abrahams and Braund (Citation2012); Hendrix, Eick and Shannon (Citation2012).
Jaques (Citation2000) notes that this type of SRP allows students to manipulate representations of scale, time, and space. The chemistry content in the present study is organic reaction mechanism, where particles react with each other via a series of interactions. These interactions involve movement of electrons and attraction and repulsion of charge. This lends itself to SRP in which students take on the roles of micro, particulate entities and move their bodies to represent the course the reaction takes. These otherwise invisible, rapid, reactions can then become manifest in an accessible way and, as McGregor and Anderson (Citation2023) report, this brings ‘into reality the invisible reactions that students can’t see with the naked eye’. For clarity, and consistency, the term SRP (simulation-role-play) (Aubusson et al. Citation1997) will be used, where appropriate, in this article.
Theoretical model to support the use of drama in science education
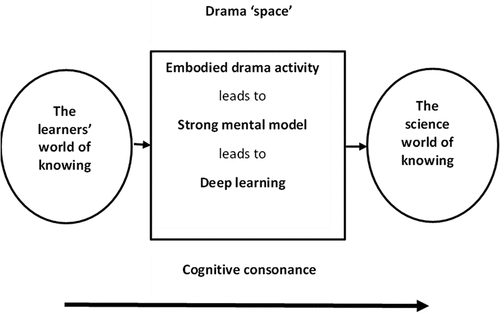
Braund (Citation2015) presented a model for the learning of science based upon the idea that science education needs to move the learner from ‘the learner’s world of knowing’ to ‘the science world of knowing’ (p.110), calling the gap between the two the ‘experiential space’ (p.110). This gap may be small or even partly overlap. When drama is the pedagogy linking the two worlds, he renames the experiential space as the ‘drama space’ (p.111) as seen in .
Figure 2. A model of learning science through drama (Braund Citation2015, p.111).
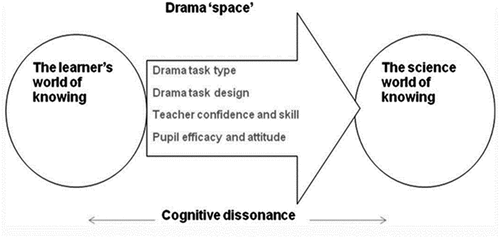
The theory of embodied cognition (Varela, Thompson, and Rosch Citation2017) emphasises the role of the body and its interaction with the external environment in eliciting cognitive activity and, in that sense, drama can provide that embodied experience to trigger cognition. Furthermore, Craick and Watkins (Citation1973) concluded that both maintenance rehearsal (simple, rote practice) and elaborative rehearsal (making connections with context and prior knowledge) are necessary for short-term memory and the latter, contributes to an improvement of memory performance. In the study reported here there is rote practice in the form of practice examination questions and more elaborative rehearsal in the form of drama. This drama links complex body-based movement as representations of micro particles, with oral and aural representations of chemical reactions providing the opportunity for deeper processing to occur. This is consistent with Scott, Harris and Rothe (Citation2001) who reported the use of dramatizing action aids memory.
Dorion (Citation2009) stated that drama is a pedagogical approach, effective in encouraging learning for concepts relying on complex analogies. Chemistry education depends on an appreciation of sub-micro worlds not visible to the student, and yet, in order to make sense of organic reaction mechanisms, they will need some personal understanding of three-dimensional shapes of atoms, molecules and ions, their interactions with each other, the movement of electrons and fluidity of charge as reactions progress. Use of SRP invites the student to step out of the everyday world into alternative worlds of their own construction, to produce their own personalised analogies for the unseen. This is done by the use of the body, in conjunction with imagination, to represent the interaction of reactant species. This micro, hitherto unexplored, scientific world becomes the newly constructed world of the individual. The provision of a script ensures the chemistry holds true, but the interpretation and understanding through somatic experience lead to reinventing the individual understanding of the students. This transformation of worlds, Rasmussen (Citation2010) claims, is realised by the ability of drama to transform a learner’s experiences through the recognition of new shapes and forms. Courtney (Citation1989) uses the term as if, describing ‘the transformation of being into something else; turning the actual into the fictional in order to work with it’ (p.14). This transformation allows the learner to personally make sense of the science presented in a script. This sense making can serve to bridge the gap between the learner’s world of knowing and the science world of knowing.
These acts of transformation are brought about via a relationship that exists between the imagined and the real worlds that Boal (Citation1979) refers to as ‘metaxis’ (p.74); the interface between the participant and their fictitious world. Here the actor is able to make sense of the world at both levels simultaneously and can create alternative understandings or ideas within which the drama is situated. This parallels ideas by Edmiston (Citation2003) in which the everyday world is thought of in terms of a ‘what is’ (IS) reality of the student and the ‘what if’ (IF) reality of the drama world of the imagination. When using drama Edmiston (Citation2003) proposes that students enter a dual reality of IS+IF where the new reality is mediated by the everyday and drama worlds, providing a more nuanced framework through which to view the subject under exploration. These applications might lend themselves to the exploration of scientific chemistry concepts where the behaviour of sub-micro particles can be reimagined (IF) in the context of the school laboratory or classroom (IS) to give the IS+IF condition for learning to take place.
Challenges facing students in learning about organic reaction mechanisms
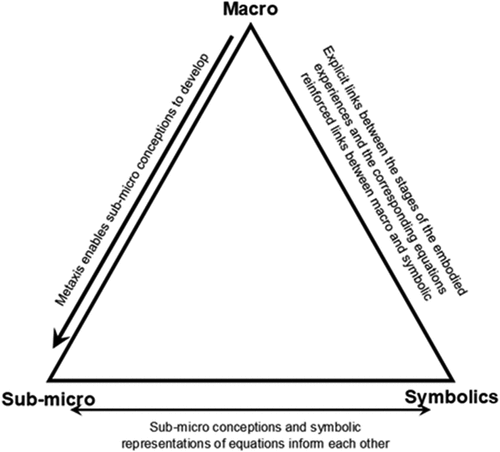
Organic chemistry forms a large part of the post compulsory (aged 16–18 years) chemistry curriculum in the UK. Ellis (Citation1994) identified three main difficulties for students in organic chemistry; there are no problem-solving algorithms, there is need for three-dimensional thinking, and there is an extensive new technical vocabulary to be mastered. Furthermore, Grove, Cooper and Rush (Citation2012) suggested visualisation is key to learning organic chemistry and that students need to be able to represent atoms, molecules, ions, and electrons and to learn to translate and navigate between different models in meaningful ways. This requires students to navigate fluently between the macro, sub-micro, and symbolic levels of thinking (Johnstone Citation2010) as represented in . When applied to chemistry, macro level thought relates to real-world experiences, such as carrying out practical work, sub-micro relates to the particulate invisible world and symbolics refers to the representations made of the sub-micro level, such as written equations.
Figure 3. Triangle of multi-level thought in science, based on Johnstone (Citation2010, p. 24).
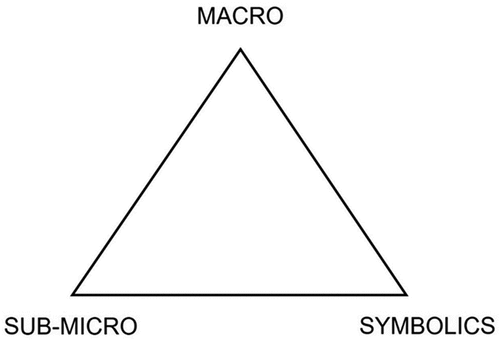
Organic reaction mechanisms are considered to be one of the most challenging areas in the post 16 chemistry curriculum (O’Dwyer and Childs Citation2011). These mechanisms are a way of representing the stages of a chemical reaction by considering the movement of electrons to make and break bonds, ultimately leading to the formation of chemical products, often via a series of intermediate stages. Students are expected to work with both two-dimensional written (symbolics) and three-dimensional mental representations of particles (sub-micro) (Adnan, Hill and Reid (Citation2004)).
Chemical reactions can be considered to consist of a number of sequential steps, each dependent on the outcome of the previous one. The generally accepted method of generating and representing these steps, in a two-dimensional form, uses electron pushing formalisation (EPF), developed by Kermack and Robinson (Citation1922). Here, electron movement in each step of a reaction can be represented on paper using curly arrows, with the direction of each arrow signifying movement from areas of high electron density to areas of low electron density. The sequence of steps from start to finish is called a reaction mechanism, as exemplified in , one of the many hundreds of examples students may encounter. The mechanistic thinking required of chemistry students involves an appreciation that each complete mechanism comprises a series of steps, each one contingent on the last; logical reasoning allows the student to determine what will happen at each stage until the final product in a reaction has been reached. Each reaction mechanism can then be presented on paper as a representation of these steps.
Figure 4. Example of a simple organic reaction mechanism representing the nucleophilic substitution of bromomethane.
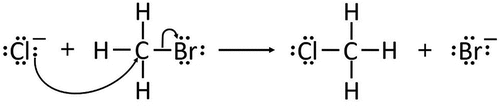
Working with first year chemistry undergraduates Bhattacharyya and Bodner (Citation2005) concluded that the curly arrows used in EPF held no physical meaning for many of the undergraduates, indicating a lack of understanding of the role of this formalisation in explaining how and why a reaction takes place. That is, the undergraduates did not recognise curly arrows as a means of predicting or explaining a reaction. One undergraduate went as far as saying ‘It’s basically playing around; I’ll try and force it to work; it gets me to the product’ (Bhattacharyya and Bodner Citation2005, 1405). Similarly, Ferguson and Bodner (Citation2008) reported that the process of drawing curly arrows was mechanical and had little intrinsic meaning for students. There was a heavy reliance on memorising specific reactions rather than developing an understanding of how to work out the relevant reaction mechanism. This dependence on memorisation is a commonplace strategy in school (O’Dwyer and Childs Citation2011) and university (Ferguson and Bodner Citation2008) when working with organic reaction mechanisms.
Grove, Cooper and Rush (Citation2012) reported that, depending on the reaction, between 30% and 60% of the 300 undergraduates in their study did not attempt to write any mechanisms, and that 15–20% added in curly arrows after predicting a reaction product. Furthermore, Grove, Cooper and Cox (Citation2012) reported that undergraduates felt it unnecessary to use, or were unable to engage with, the construction of reaction mechanisms via EPF. For more complex reactions students that used mechanisms scored statistically significantly higher than those that did not, and Salame, Casiona and Hodges (Citation2020) proposed that reliance on memorisation, as opposed to conceptualisation, would be a hinderance to understanding. This reinforces the importance for students to develop fluency in generating reaction mechanisms right from the introduction of the topic. Since the number and complexity of mechanisms increases as studies progress, it is impossible for students to memorise the thousands of reaction mechanisms needed. This, coupled with the lack of problem-solving algorithms (Ellis Citation1994), means an understanding of the nature of reaction mechanisms is necessary to advance their studies.
For students to function as experts in this area of chemistry, they will need to navigate Johnstone’s (Citation2010) triangle with ease and rely less on memorization in order to realise examples rationally. Stricklanda, Kraft and Bhattacharyya (Citation2010) propose that to use Johnstone’s (Citation2010) triangle in understanding reaction mechanisms requires strong mental models. Within this article mental models are taken to be mental images of objects (Reisberg Citation1997) that can be stored and manipulated in the absence of their physical manifestation (Hegarty and Waller Citation2005). In science education there is a reliance on external models to produce strong mental models Touli, Talbi, and Radid (Citation2012), and these may be symbolic, verbal, or gestural (Gilbert Citation2007). Ong and Hodges (Citation2010) found that observational learning alone did not lead to an updating of the internal mental model to the same extent as embodied learning had done.
SRP, as a form of embodied learning, could therefore be utilised in the production of strong mental models in the teaching and learning of organic reaction mechanisms.
Materials and methods
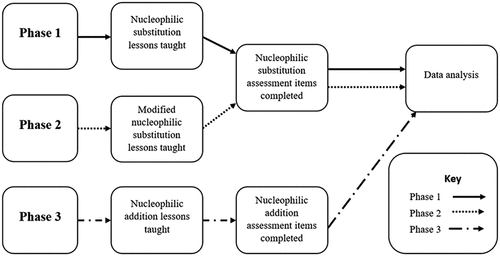
The research question in this study asks ‘Do students’ marks, in A Level examination question on organic reaction mechanisms, differ, in a statistically significant manner, depending on whether they have been taught using drama (in the form of SRP) or practice examination questions?’ This question allowed for the collection of quantitative data in the form of marks awarded for students’ answers to authentic A Level questions. In order to operationalise this, agreement was obtained from seven 11–18 schools and one Further Education college, all in the North of England. To be eligible a school or college, needed to have two A Level chemistry classes running concurrently in the first year of the A Level chemistry course at the start of the study, and not have had any teaching of organic reaction mechanisms prior to this time on their A Level studies.
The study used a quasi-experimental design (Siegle Citation2019) as students could not be randomly allocated to the drama, or non-drama, classes in each school, but had to remain within their usual teaching classes. As organic reaction mechanisms are not studied pre-A Level (i.e. before age 16 years) in the English science curriculum, it was judged there was no need for pre-testing. Intervention lessons were designed in which the input variable was the teaching and learning pedagogy and the output variable were the marks awarded in response to student answers to examination questions (subsequently referred to as assessment items). The study consisted of three phases (see ). During each phase of the intervention one group of students was taught the new chemistry content using drama while the other group used practice examination questions to study the same content. The learning outcomes for each lesson were the same for the two groups and appropriate to each phase of the study. Following the intervention lessons, students in both groups all answered the same assessment items, in the form of A Level chemistry past paper questions, since this is the method of assessment that will be applied externally at the end of the A Level course. Responses to the assessment items were marked and the resultant scores analysed quantitatively. In each of the phases the intervention and control groups took part in a one-hour lesson taught by the researcher (a highly experienced chemistry teacher).
Ethical approval was given by the University of Leeds (reference AREA 14–038), with all participants giving written consent for participation in the study and for the anonymised data to be used for research. To ensure no student in the study was disadvantaged the researcher offered, after research data was collected, to reteach the drama lesson for the non- drama group and vice-versa.
Phase 1
Phase 1 consisted of students from seven different schools and one FE college in the first year of their A Level studies, n(drama) = 81, n(non-drama) = 89. In each institution one class was randomly allocated as the drama and the other the control class. Specification statements relating to the topic of nucleophilic substitution were identified; allowing clear learning outcomes to be defined and these were subsequently used to inform lesson planning. The reaction examples, used in the teacher-led start to the lesson, introduced the new chemistry and were kept the same for both drama and control classes with the consolidation and demonstration of learning allocated the same length of lesson time, irrespective of the group. The intervention lessons involved the use of SRP in which the students worked in small groups to consolidate and demonstrate their learning by transforming a pre-written script into a play performed to the rest of their peers. The use of the script ensured the chemistry remained aligned with that required by the examination board; a number of props were provided for each reaction to be enacted. A sample of such can be seen in .
Figure 6. A sample of a kit of ‘props’ provided to use when representing one of the reaction mechanisms, typical of those used in phases 1 and 2 of the study.

Student agency rested in their ability to use their bodies to represent the movement of electrons and the formation of products. Agency was given to students to reinvent themselves as atoms and ions with signage, to self-identify as nucleophiles with corresponding use of postures and facial attitudes, to use pompoms to represent electrons and their movement e.g. taking a pompom ‘electron’ from another ‘particle’, using rulers with pompoms stuck to them to represent bonds being made or broken and sticky plus and minus signs to represent the outcomes of charge moving in the ‘human system’. Some groups made use of large arrows to show the direction of movement of electrons, while others used their body shapes and movement. Students were free to use as much of the kit as they chose and worked in small groups to choreograph the reaction before presenting this to their peers and writing the mechanism into their own notes.
This aligns with the more structured end of the continuum proposed by Ødergaard (Citation2003) and places the SRP towards the student, as opposed to teacher, end of the continuum. The non-drama group used practice examination questions as an alternative form for consolidation and demonstration of understanding.
Approximately two weeks after their lessons, all students completed the relevant assessment items.
Phase 2
Whilst it was anticipated that all of the schools in Phase 1 would continue into a second year of the study this was not the case. Phase 2 was therefore a small-scale repeat of phase 1, working with four of the 11–18 schools, n(drama) = 32, n(non-drama) = 34. This allowed for students to be followed into their second year of study (Phase 3). Example reactions used in the teacher introduction were different from Phase 1, due to changes in the curriculum in the intervening year.
Phase 3
Phase 3 followed Phase 2 students from the same four schools used in Phase 2 into the second year of their studies. n(drama) = 7, n(non-drama) = 17. These reduced numbers were a consequence of students not continuing their studies into the second year, additionally some students moved classes to fit in with school timetabling restrictions. The same format for lesson planning was used as in Phases 1 and 2 for the new chemistry content relating to nucleophilic addition.
Student agency in Phase 3 was higher than in Phases 1 and 2, being more student directed and with a higher degree of spontaneity. The only limitation placed on the students were that the SRP needed to represent a series of chemical points given to them to ensure the relevant examination board statements were addressed. This is in line with McGregor and Anderson (Citation2023) who acknowledge that for specific scientific concepts to be explored it may not be appropriate to give full student autonomy.
What form the enactment took, and how students used their body shapes, facial expressions and movement was left to them to decide. The enactments presented to their peers ranged from representations of friendships being made and broken to scripts that mirrored the scientific vocabulary of the mechanism. A range of props () were provided with the option for students to use as they required. The non-drama group used content appropriate practice examination questions for consolidation and demonstration of understanding.
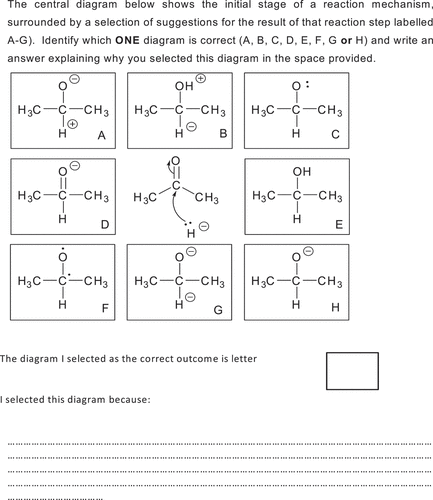
In addition to the past examination questions, Phase 3 assessment items included a diagnostic question that had been agreed, by two academics in the field of chemistry education, to need a deep level of understanding to answer correctly. Each possible answer was included as a result of discussions with chemistry educators and consulting the relevant literature. All answers, except for the correct one, were judged to be representative of answers resulting from misconceptions ().
Figure 8. Diagnostic question used in phase 3 of the study. Otter (Citation2020, p. 368).
Results
Quantitative data analysis
Student written responses were marked in two ways. Firstly, marks were awarded using the examination board mark scheme. Secondly, in order to analyse whether or not there were any differences in performance in terms of meeting these individual criteria, a more detailed, fine grain, mark scheme was produced. The latter arose because in some cases a number of criteria needed to have been fulfilled before a single mark could be awarded in the examination board mark scheme.
For example, as seen in an extract of the coding schedule in , for Mark 4 to be awarded using the examination board mark scheme three things need to have been correctly carried out; the arrow needs to be moving in the correct direction (from source to sink), needs to be at a prescribed distance from the source atom, and needs to be within the prescribed distance from the sink atom. In the fine grain mark scheme, subject to the first criterion above being fulfilled, the second and third marks can gain credit if there is some degree of imprecision, allowing for partially correct answers to be credited.
Table 1. An example of how the examination board mark scheme and the fine grain mark scheme are linked.
To reduce potential for researcher bias, the fine-grain mark scheme was scrutinised by a PhD chemist and an academic chemistry educator.
To ensure consistency of application of the mark schemes, a sample of student scripts were marked by the researcher and a second chemistry educator. Inter-marker reliability was found to be high for both both the schemes. The inter-class correlation for the total marks awarded using the examination board mark scheme was statistically significant using an ANOVA (F(18,18) = 62.84, p < 0.001). The inter-class correlation for the marks for the total fine-grain marks was also statistically significant (F(18, 18) = 123.25, p < 0.001). For Phase 3, a sample of student scripts were marked by the researcher and a practicing A Level chemistry teacher. Using an ANOVA test inter-marker reliability was found to be high for both mark schemes. For the examination board mark scheme: F(8,8) = 62.84, p < 0.001 and for the fine grain mark scheme: F(8, 8) = 0.977, p < 0.001.
Phase 1 data analysis
Student data from Phase 1 was parametric: n(drama) = 81, n(non-drama) = 89, and statistical tests were selected accordingly. To ensure comparability of the two groups, the predicted A Level chemistry grades for students were tested for differences between the two groups, using an independent samples t-test. This revealed a statistically significant difference between the predicted grades of students in the two groups: t (168) = 2.36, p = 0.19.
To eliminate this factor as a covariant, a regression ANCOVA test was conducted to determine whether there was a difference between the drama and non-drama groups on the exam scores, controlling for predicted A Level grade. There was no statistically significant difference between the marks awarded to the two groups (drama and non-drama) when using the examination board mark scheme, after controlling for predicted A Level grade: F(1,169) = 0.18, p = 0.68 nor when the fine grain mark scheme was used: F(1,169) = 0.11, p = 0.75.
Phase 2 data analysis
Student data from Phase 2 was also parametric: n(drama) = 32, n(non-drama) = 34. To ensure comparability of the two groups, the predicted A Level chemistry grades for students were tested for differences between the two groups, using an independent samples two tailed t-test. There was no statistically significant difference between the predicted grades of the two groups (drama and non-drama groups): t(64) = 1.05, p = 0.30.
Using independent samples two tailed t-test, it was found there was no statistically significant difference between marks awarded to the two groups (drama and non-drama) when using the examination board mark scheme: t(64) = −1.12, p = 0.27 or the fine grain mark scheme: t(64) = −1.57, p = 0.12.
Phase 3 data analysis
Data for Phase 3 were judged to be non-parametric and the smaller amount of data (n (drama) = 7, n (non-drama) = 17) meant the only analysis carried out was the difference between the group mark using both mark schemes. A Mann-Whitney U test was performed to evaluate whether there was a difference between the scores obtained by the drama and non-drama groups. There was no statistically significant difference between marks awarded to the two groups (drama and non-drama) when using the examination board mark scheme: U = 46.00, p = 0.39, nor the fine grain mark scheme: U = 40.50, p = 0.22.
Whilst the small sample size means that the results should be viewed with caution it was found that a statistically significantly higher proportion of the drama group answered the diagnostic question correctly compared to the non-drama group: z = 2.20, p = 0.03% correct in drama group is 71% and in the non-drama group is 24.%.) and this corresponds to a large effect size.
Answer E, representing the final product of the reaction rather than the intermediate, was selected by 24% of the non-drama group, and 29% of the drama group. This answer indicates these students are not thinking mechanistically, instead they are thinking of the final product of the reaction, which is not what is required here. This is consistent with the findings of Bhattacharyya and Bodner (Citation2005) who argue that students unskilled in using reaction mechanisms focus on the final product as opposed to how a logical reaction mechanism will progress step-wise to yield the final reaction product.
Differences occurred between the two groups with the remainder of the answers. Those from the non-drama group were distributed between H and C, including a 47% response rate to answer C with a 0% uptake of this answer from the drama group. This answer demonstrated their ability to visualise the cause and effect of the movement of electrons to produce an intermediate in this reaction, but did not demonstrate an appreciation of the resultant changes to charge distribution.
Responses from the two groups to answer, H, varied with a 71% selection rate for the drama group and 20% uptake for the non-drama group. This answer demonstrated an ability to visualise the cause and effect of the movement of electrons to produce an intermediate in this reaction, including an understanding of the impact of moving charge.
Discussion
As discussed earlier, students need to be able to fluently navigate the macro, sub-micro, and symbolic levels of thinking in Johnstone’s (Citation1991) triangle in order to have fluency in working with organic reaction mechanisms. What follows is an interpretation of how the two pedagogies above, SRP and completion and marking of practice examination questions, link to these three aspects of Johnstone’s triangle.
Accessing aspects of Johnstone’s triangle in the drama and non-drama lessons
The real-world experiences of the SRP in the drama group lessons enable students to access the chemistry in a concrete embodied way, and to construct what Taber (Citation2013) refers to as macroscopic conceptualisations at a descriptive level. The invisible sub-micro world can be accessed through the use of embodied learning; the macro experiences providing a conduit to ‘metaxis’ (Boal Citation1979, 74) allowing students to create their own transformations in the sub-micro world with its associated understandings. The following student statement illustrates how the judicious use of props helped the macro and micro world come together;
‘They [pompoms] were stuck in different places weren’t they, cos as well as pairs they were on the sheet whereas in bonds they were on rulers’ (student P, phase 2, school 5)
An embedded aspect of the SRP was the use of appropriate technical language and the writing of the reaction mechanisms (symbolics), introducing clear links between the levels of Johnstone’s (Citation1991) triangle. As one student explained:
‘I think the 3D [macro experiences] just helped secure the understanding of what was going on and then looking at the 2D[symbolics] so you could actually write it down’. (student E, phase 1, school 5)
Another student explained how the drama had provided a stimulus to answer the assessment items, linking all three aspects of Johnstone’s (Citation1991) triangle.
‘I thought about them [pompoms] and then sort of thinking about that in my mind helped me when I was writing down [answers to exam questions] to sort of go over it, yes, they moved there, rather than just sort of going straight to it [the exam question]’. (student E, phase 1, school 4)
These emergent relationships between the three aspects of the Johnstone triangle in the drama lesson are represented in .
Figure 9. The relationship between the macro, sub-micro, and symbolic levels in the drama lessons and how they support each other to strengthen conceptual understanding. Adapted from Johnstone (Citation2010, p. 24).
The integration of the macro, sub-micro and symbolic aspects of the student experiences in the drama group seem to allow students to develop IS+IF referred to by Edmiston (Citation2003).
With the non-drama group lessons there were no embodied macro experiences and so the macro dimension of conceptualisation was reduced, providing no opportunities for ‘metaxis’ (Boal Citation1979, 74). The absence of the IF (Edmiston Citation2003) dimension resulted in less robust understandings at the sub-micro level.
The links between developing aspects of Johnstone’s triangle and the construction of mental models
In this study students’ mental models find expression in the form of written responses to a range of assessment items consisting of examination questions and a diagnostic question. Students need to successfully engage with symbolic two-dimensional representations of reactions presented in the form of examination questions, link those to the sub-micro world of what is occurring at a particulate level and then retranslate these ideas into two-dimensional written answers.
During drama lessons students worked with macro, sub-micro and symbolic interpretations, whilst those in the non-drama lessons worked with sub-micro and symbolic representations. Based on the statistical findings it would appear that both pedagogies are effective for teaching and learning to facilitate answering A Level examination questions.
Although students in the drama group had not practiced examination questions as part of their intervention lessons, it appears they had the ability to answer them at least as well as the students in the non-drama group.
Answering the diagnostic question
In order to answer the Phase 3 diagnostic question successfully, students need to have formed an appreciation of the three-dimensional geometry of the reactants from the two-dimensional representation in the question and use this to determine how the reaction will proceed to an intermediate, a novel application neither group had addressed in the intervention or control lessons. This intermediate structure then needs to be mentally transformed into a two-dimensional format in order to select the correct representation from those presented. A student therefore needs to fluently navigate between the sub-micro and symbolic representations of chemistry as exemplified by Grove, Cooper and Rush (Citation2012) and Johnstone’s (Citation1991, Citation2010). These practices have a high level of demand and depend upon strong sub-micro conceptualisations. How well a student answers the novel demand of the Phase 3 diagnostic question will depend upon the strength of the mental model formed, since recall will not help in this new context.
It appears that there is a difference between the quality of learning produced through the use of drama as opposed to the completion and marking of examination questions, since the drama group outperformed the non-drama group in the Phase 3 diagnostic question in a statistically significant way. We propose the learning experience of SRP contributed to the construction of strong mental models: the embodied aspect of the lessons contributing to conceptualisation of the macro dimension, so promoting a strong IS+IF paradigm. This embodied aspect was absent in the non-drama group. As mental models have spatial, physical and kinaesthetic dimensions (Hostetter and Alibali Citation2008) we propose that embodied learning, also possessing these dimensions, contributed to the production of strong mental models by the drama students, through the development of the descriptive level conceptualisation of the reaction mechanisms, to a greater extent than the practising and marking of examination-style questions. Additionally, the merging of real and imagined worlds through the use of SRP contributed to the development of stronger sub-micro, explanatory level, conceptualisation of the reaction mechanisms than the practicing and marking of examination questions.
This would indicate that as students move from concrete macro learning, adding more abstract sub-micro understanding of the relevant chemical concepts, they are transitioning from surface to deep learning. Hattie and O’Donoghue (Citation2018) proposed two categories of learning; surface ‘factual and content’ (p.98), and deep ‘integrated and relational’ (p.98). Students successfully completing the diagnostic question were explaining, synthesising and evaluating information in a novel context, integrated their ideas to determine an intermediate for a chemical reaction. This is a complex set of processes, characteristic of deep learning. Drawing on the work of Tregaust, Chittleborough and Mamiala (Citation2003) it can be said that the use of SRP has promoted the development of relational, deep, understanding via mental integration of the three aspects of Johnstone’s (Citation1991) triangle, when applied in the context of organic reaction mechanisms. These processes resulted in a larger proportion of students in the drama group developing robust mental models than did those in the non-drama group. A representative example of this can be seen in the following student comment:
‘I think when we write our mechanisms and draw all the arrows, it’s quite hard to visualise it, like understand what’s happening. So, when we did the drama, it was quite good to really understand what’s happening and why the arrows go where they do’.(student G, phase 2, school 1)
Figure 10. A model for learning science through drama, based on a modified Braund (Citation2015, p. 111).
Conclusion
The findings reported here, in addition to expanding the age range of students for which SRP can be effectively used, support the literature claiming that drama can be used to successfully teach challenging science topics (Arieli Citation2007; Braund and Ahmed Citation2018). SRP is at least as effective in promoting learning of organic reaction mechanisms than a more traditional approach. The findings also support claims advanced by Metcalfe et al. (Citation1984), that deeper understanding of scientific ideas is significantly increased following drama intervention. This deeper level understanding of organic reaction mechanisms has been proven to be necessary for students to succeed at undergraduate level (Salame, Casiona, and Hodges Citation2020), therefore it appears advantageous to use SRP as a pedagogy with this age range, for this topic.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abed, O. 2016. “Drama-Based Science Teaching and Its Effect on pupils’ Understanding of Scientific Concepts and Their Attitudes Towards Science Learning.” International Education Studies 9 (10): 163–173. https://doi.org/10.5539/ies.v9n10p163.
- Abrahams, I., and M. Braund, eds. 2012. Performing Science: Teaching Chemistry, Physics and Biology Through Drama. London: Bloomsbury.
- Adnan, K., R. Hill, and N. Reid. 2004. “Ideas Underpinning Success in an Introductory Course in Organic Chemistry.” University Chemical Education 8 (2): 40–51.
- Alibali, M. W., and M. J. Nathan. 2012. “Embodiment in Mathematics Teaching and Learning: Evidence from Learners’ and Teachers’ Gestures.” Journal of the Learning Science 21 (2): 247–286. https://doi.org/10.1080/10508406.2011.611446.
- Arieli, B. 2007. “The Integration of Creative Drama into Science Teaching.” PhD diss., University of Kansas.
- Aubusson, P., S. Fogwill, L. Perkovic, and B. Rajender. 1997. “What Happens When Pupils Do Simulation-Role-Play in Science?” Research in Science Education 27 (4): 565–579. https://doi.org/10.1007/BF02461481.
- Baskerville, D., D. McGregor, and A. Bonsall. 2023. “Re-Thinking Theorising About the Use of Drama, Theatre and Performance in Learning science’.” In Learning Science Through Drama. Exploring International Perspectives, edited by D. McGregor and D. Anderson, 11–26. Switzerland: Springer.
- Bhattacharyya, G., and G. Bodner. 2005. “It Gets Me to the Product’: How Pupils Propose Organic Mechanisms.” Journal of Chemical Education 82 (9): 1402–1407. https://doi.org/10.1021/ed082p1402.
- Boal, A. 1979. Theatre of the Oppressed. London: Pluto Press.
- Braund, M. 2015. “Drama and Learning Science: An Empty Space?” British Educational Research Journal 41 (1): 102–121. https://doi.org/10.1002/berj.3130.
- Braund, M., and Z. Ahmed. 2018. “Drama As Physical Role-Play: Actions and Outcomes for Life Science Lessons.” South Africa, Journal of Biological Education 53 (4): 412–421. https://doi.org/10.1080/00219266.2018.1490799.
- Courtney, R. 1989. Play Drama and Thought: The Intellectual Background to Dramatic Education. 4th ed. Toronto: Simon and Pierre Publishing.
- Craick, F., and M. Watkins. 1973. “The Role of Rehearsal in Short-Term Memory.” Journal of Verbal Learning and Verbal Behavior 12 (6): 599–607. https://doi.org/10.1016/S0022-5371(73)80039-8.
- Dawson, E., A. Hill, J. Barlow, and E. Weitkamp. 2009. “Genetic Testing in a Drama and Discussion Workshop: Exploring Knowledge Construction. Research in Drama Education.” Research in Drama Education: The Journal of Applied Theatre and Performance 14 (3): 361–390. https://doi.org/10.1080/13569780903072174.
- Dorion, K. R. 2009. “Science Through Drama: A Multiple Case Exploration of the Characteristics of Drama Activities Used in Secondary Science Lessons.” International Journal of Science Education 31 (16): 2247–2270. https://doi.org/10.1080/09500690802712699.
- Edmiston, B. 2003. “What’s My Position? Role, Frame and Positioning When Using Process Drama.” Research in Drama Education 8 (2): 221–230.
- Ellis, J. 1994. “How Are We Going to Teach Organic Chemistry if the Task Force Has Its Way.” Journal of Chemical Education 71 (5): 399–403. https://doi.org/10.1021/ed071p399.
- Ferguson, R., and G. Bodner. 2008. “Making Sense of the Arrow Pushing Formalism Among Chemistry Majors Enrolled in Organic Chemistry.” Chemical Education Research and Practice 9 (2): 102–113. https://doi.org/10.1039/B806225K.
- Gilbert, J. K., ed. 2007. Visualization in Science Education. Dordrecht: Springer.
- Grove, N., M. Cooper, and E. Cox. 2012. “Does Mechanistic Thinking Improve Pupil Success in Organic Chemistry?” Journal of Chemical Education 89 (7): 850–853. https://doi.org/10.1021/ed200394d.
- Grove, N., M. Cooper, and K. Rush. 2012. “‘Decorating with Arrows: Towards the Development of Representational Competence in Organic Chemistry.” Journal of Chemical Education 89 (7): 844–849. https://doi.org/10.1021/ed2003934.
- Hattie, J. A., and G. M. O’Donoghue. 2018. “A Model of Learning: Optimising the Effectiveness of Learning Strategies.” In Contemporary Theories of Learning: Learning Theorists in Their Own Words, edited by K. Illeris. 2nd, 97–113. London: Routledge.
- Hegarty, M., and D. Waller. 2005. “Individual Differences in Spatial Abilities.” In The Cambridge Handbook of Visuospatial Thinking, edited by P. Shah and A. Miyake, 121–169. Cambridge: Cambridge University Press.
- Hendrix, R., C. Eick, and D. Shannon. 2012. “The Integration of Creative Drama in an Inquiry-Based Elementary Program: The Effect on Pupil Attitude and Conceptual Learning.” Journal of Science Teacher Education 23 (7): 823–846. https://doi.org/10.1007/s10972-012-9292-1.
- Hostetter, A. B., and M. W. Alibali. 2008. “Visible Embodiment: Gestures As Simulated Action.” Psychonomic Bulletin and Review 15 (3): 495–514. https://doi.org/10.3758/PBR.15.3.495.
- Jaques, D. 2000. Learning in Groups: A Handbook for Improving Group Work. London: Routledge.
- Johnstone, A. 1991. “Why Is Science Difficult to Learn? Things Are Seldom What They Seem.” Journal of Computer Assisted Learning 7 (2): 75–83. https://doi.org/10.1111/j.1365-2729.1991.tb00230.x.
- Johnstone, A. 2010. “You Can’t Get There from Here.” Journal of Chemical Education 87 (1): 22–29. https://doi.org/10.1021/ed800026d.
- Kermack, W., and R. Robinson. 1922. “An Explanation of the Property of Induced Polarity of Atoms and an Interpretation of the Theory of Partial Valencies on an Electronic Basis.” Journal of the Chemical Society 121:426–440. https://doi.org/10.1039/CT9222100427.
- Marsella, S. C., W. L. Johnson, and C. LaBore 2000. “Interactive Pedagogical Drama.” In: Fourth International Conference on Autonomous Agents, 3-7 June 2000Barcelona. 3: 301–308.
- McGregor, D. 2012. “Dramatising Science Learning: Findings from a Pilot Study to Re-Invigorate Elementary Science Pedagogy for Five- to Seven-Year Olds.” International Journal of Science Education 34 (8): 1145–1165. https://doi.org/10.1080/09500693.2012.660751.
- McGregor, D., and D. Anderson. 2023. “Epistemic Contrasts of Different Drama Conventions: A Content Analysis of the Range of Affordances Variations of Role Play Offer for Learning Science.” In Learning Science Through Drama, Edited by Exploring International Perspectives, edited by D. McGregor and D. Anderson, 43–67. Switzerland: Springer.
- Metcalfe, R. J., S. Abbott, P. Bray, J. Exley, and D. Wisnia. 1984. “Teaching Science Through Drama: An Empirical Investigation.” Research in Science and Technological Education 2 (1): 77–81. https://doi.org/10.1080/0263514840020109.
- Ødergaard, M. 2003. “Dramatic Science: A Critical Review of Drama in Science Education.” Studies in Science Education 39 (1): 75–101. https://doi.org/10.1080/03057260308560196.
- O’Dwyer, A., and P. Childs 2011. “Second Level Irish Pupils’ and Teachers’ View of Difficulties in Organic Chemistry.” Paper presented at the International Organisation for Science and Technology Education Symposium, Reading, June 20-21.
- Ong, N. T., and N. J. Hodges. 2010. “Absence of After-Effects for Observers After Watching a Visuomotor Adaptation.” Experimental Brain Research 205 (3): 325–334. https://doi.org/10.1007/s00221-010-2366-4.
- Otter, C. A., 2020. “The Use of Drama in A Level Chemistry: A study into the effects of simulation-role-play on the quality of, and student attitudes towards, learning of organic reaction mechanisms.” PhD diss., University of Leeds.
- Rasmussen, B. 2010. “The ‘Good Enough’ Drama: Reinterpreting Constructivist Aesthetics and Epistemology in Drama Education.” Research in Drama Education 15 (4): 529–554. https://doi.org/10.1080/13569783.2010.512187.
- Reisberg, D. 1997. Cognition. New York: Norton.
- Salame, I., P. Casiona, and N. Hodges. 2020. “Examining Challenges That Students Face in Learning Organic Chemistry Synthesis.” International Journal of Chemistry Education Research 4 (1): 1–9. https://doi.org/10.20885/ijcer.vol4.iss1.art1.
- Scott, C., R. Harris, and A. Rothe. 2001. “Embodied Cognition Through Improvisation Improves Memory for a Dramatic Monologue.” Discourse Processes 31 (3): 293–305. https://doi.org/10.1207/S15326950dp31-3_4.
- Siegle, D. 2019. Educational Research Basics: Experimental Design. University of Connecticut. Accessed November 26, 2022. https://researchbasics.education.uconn.edu/experimental-_research/#.
- Stricklanda, A. M., A. Kraft, and G. Bhattacharyya. 2010. “What Happens When Representations Fail to Represent? Graduate Pupils’ Mental Models of Organic Chemistry Diagrams.” Chemistry Education Research and Practice 11 (4): 293–301. https://doi.org/10.1039/C0RP90009E.
- Taber, K. 2013. “Revisiting the Chemistry Triplet: Drawing Upon the Nature of Chemical Knowledge and the Psychology of Learning to Inform Chemistry Education.” Chemistry Education Research and Practice 14 (2): 156–168. https://doi.org/10.1039/C3RP00012E.
- Touli, E. H., M. Talbi, and M. Radid. 2012. “‘Teaching-Learning of Chemistry: Analysis of Representations of Learners on the Modelling of Chemical Transformation.” Procedia - Social & Behavioral Sciences 46:47–52. https://doi.org/10.1016/j.sbspro.2012.05.065.
- Tregaust, D., G. Chittleborough, and T. Mamiala. 2003. “The Role of Sub-Microscopic and Symbolic Representations in Chemical Explanations.” International Journal of Science Education 25 (11): 1353–136. https://doi.org/10.1080/0950069032000070306.
- Varela, F., E. Thompson, and E. Rosch. 2017. The Embodied mind, Revised edition: Cognitive Science and Human Experience. London: MIT press.