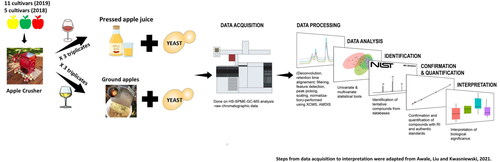
Abstract
Cider is often produced from difficult-to-grow heirloom apple cultivars not commonly produced in North America. As such, the value of widely grown dessert apples for fermented cider was explored, with ciders produced from eleven cultivars analyzed. Additionally, the practice of maceration, or fermentation with pomace, was also investigated. Volatile compounds were analyzed by gas chromatography-mass spectrometry. Raw mass spectrometry (MS) data was then followed by an initial untargeted statistical analysis. Principal component analysis of the MS features demonstrated clear separation between cultivars and between maceration effects, prompting further investigation into the compounds impacting cultivar and maceration discrimination. The aroma-active compounds identified are known to elicit sensorial responses often described as sweet, fruity, and floral. Some compounds have been previously identified as primarily apple-derived. While esters and higher alcohols, originating from yeast metabolic activities, were also identified as contributing factors of the observed differences. Despite these compounds being impacted by yeast metabolism, they were highly influenced by cultivar and treatment, indicating apple-derived precursors were, in part, influencing the yeast-mediated aroma differences. ‘Galaxy Gala’ cider samples had the highest overall intensities of quantified volatiles in both years, driven by high eugenol and 1-butanol concentrations. Extended apple pomace maceration did not induce new aromas but increased the content of some compounds. Maceration impact was cultivar dependent, e.g., ethyl 2-methylbutyrate in the ‘Yataka Fuji’ increased 14-fold with maceration but less in other cultivars. This work demonstrates that dessert apples produce ciders with a diversity of aromas that may be further increased via maceration and are worth consideration by producers.
Introduction
Apples (Malus domestica Borkh.) are one of the most important fruit crops in temperate regions, with worldwide production exceeding 80 million tons annually.[Citation1,Citation2] Of this, 8% of the apple production is destined for processing, such as being made into ciders.[Citation3] Global cider is anticipated to hit $6.72 billion USD by 2030, with an estimated growth rate of 3.34% compared to 2021.[Citation4] Although European countries dominate production and consumption, cider sales in the United States have increased exponentially in the past twenty years, from 0.06% (sales totaling $44 million USD) of the alcoholic beverage industry in 2008 to 0.4% ($506 million USD) in recent years.[Citation5] Most of the apples grown are classified as dessert apples, while only a small portion of the production is traditional heirloom cider cultivars. Heirloom cider apples with high tannin and acid content are typically used for European-style fermented cider production but have numerous production deficiencies compared to dessert cultivars.[Citation6,Citation7] North American producers rely heavily on the more disease-resistant, higher-producing dessert apple cultivars, which in turn have greater availability and a lower price than heirloom cultivars for cider producers.[Citation8,Citation9]
While dessert apple cultivars may have production advantages compared with heirloom types, little is known about the ciders produced from dessert cultivars. As such, characterizing dessert cultivar ciders is a current area of active research. Recent research has unveiled the potential of certain dessert apples for cider production, showcasing their suitability in comparison to traditional European apple varieties. This assessment was based on biochemical traits, such as acidity, weight, juiciness, and phenolic content in selected heirloom and dessert apple cultivars.[Citation9,Citation10] While the extensive characterization of the trace flavor chemistry of ciders made from heirloom ciders has been conducted, little work has been done on ciders from dessert apples, leaving a gap in our understanding of how these cultivars could be used in cider production.
While comparable to some of their heirloom counterparts, the diverse juice characteristics among dessert apple cultivars also create options for cider makers when blending heirloom varieties with other dessert cultivars. In general, dessert apples have a higher pH, lower titratable acidity, and lower polyphenol content than heirloom apple juice, expanding the options for blending for a particular flavor profile.[Citation11] However, the aroma profile and other important cider qualities introduced by the dessert apples are mainly unexplored.[Citation4,Citation12]
Primary volatiles released from apples are one aspect that differentiates cultivar flavor, with some of these aroma-active chemicals even persisting through aging.[Citation13] However, alcoholic fermentation plays a vital role in modifying the volatile chemistry of apples and their juice to that of fermented cider. Yeast uses precursors in the apples to make a wide range of new aroma compounds while also releasing others during the catabolism of apple must.[Citation4,Citation14] The complexity of the interactions between primary fruit metabolites, yeast-generated aromas created from apple precursors, and yeast catabolism of other compounds make it critical to process fruit through fermentation to understand cultivar differences in cider.[Citation15,Citation16] Factors such as sugar content, pH, and nitrogen content not only impact yeast-produced aromas but also impact fermentation health and kinetics that induce other volatile shifts. As all the chemistry for the fermentation is derived from the fruit in a way, all aromas could be considered fruit-dependent; however, there are some primary yeast metabolites that will be created as a result of any alcoholic fermentation. Others depend on factors such as the type of fruit, cultivar, degree of ripeness, region of production, etc (). The phenotypic variation observed among different apple cultivars is huge, with differences in optimum sugar content at ripening, days to maturity, metabolites and much more.[Citation17]
Figure 1. The diagram shows the three different sources responsible for cider aroma. Source ‘A’ encompasses metabolites generated by yeast as part of primary metabolism during fermentation regardless of the starting product (i.e., would be expected from the fermentation of apples, grapes, or a pure sucrose solution). Source ‘B’ are yeast-mediated metabolites, precursors, chemicals that are changed from their original form found in the apple as part of yeast metabolism. Source ‘C’ are volatiles compounds originating from the fruit that remain unchanged in the final cider. This figure is not considered to be a comprehensive list but a visual representation of the source of the chemicals found in cider. The figure was adapted from Dzialo et al., 2017.[Citation18]
![Figure 1. The diagram shows the three different sources responsible for cider aroma. Source ‘A’ encompasses metabolites generated by yeast as part of primary metabolism during fermentation regardless of the starting product (i.e., would be expected from the fermentation of apples, grapes, or a pure sucrose solution). Source ‘B’ are yeast-mediated metabolites, precursors, chemicals that are changed from their original form found in the apple as part of yeast metabolism. Source ‘C’ are volatiles compounds originating from the fruit that remain unchanged in the final cider. This figure is not considered to be a comprehensive list but a visual representation of the source of the chemicals found in cider. The figure was adapted from Dzialo et al., 2017.[Citation18]](/cms/asset/df9ac627-4aef-4aec-9371-69329a88837f/ujbc_a_2319931_f0001_c.jpg)
Previous research has demonstrated apple pomace (composed of apple pulp, seeds, peel, and pedicels) was abundant in esters, alcohols, ketones, and terpenes, such as ethyl 2-methylbutanoate, ethyl hexanoate, 1-hexanol, and linalool.[Citation19] However, traditional cider processing utilizes clear pressed juice for fermentation. By immediately pressing the juice from the pomace, the producer limits the time when aromas and their precursors can be extracted. While pressing the pomace before fermentation is conducted to have a “cleaner” cider product, it may lower the levels of fruit-derived volatiles and reduce other precursors that yeast could utilize to create secondary aromas. Alternatively, some cider, and apple distillate producers and producers of other fermented fruit beverages opt to ferment with the pomace or skins, to increase flavor extraction.
There are various ways in which cider aroma can be evaluated, including the use of sensory analysis and analytical tools. Sensory analysis is used to elucidate the overall perception of aromas and detect nuances that may be missed by instrumental analysis.[Citation12,Citation20] However, instrumental analysis, such as gas chromatography-mass spectrometry (GC-MS), offers objective, comprehensive, quantifiable, and stable measurements. Targeted analytical analysis, which identifies and quantifies selected aroma compounds, has been used primarily for aroma characterization until recently.[Citation21,Citation22] While this is a powerful tool for aroma characterization, it also requires preselecting which compounds are important. As a result of the synergistic interactions among aroma compounds, researchers have progressively redirected their investigations from quantitatively analyzing high-content compounds to conducting more comprehensive analyses of the volatile metabolites as a whole.[Citation23,Citation24] Untargeted metabolomics-based analysis as a broader approach facilitates a more thorough understanding of the intricate reactions among these compounds. This comprehensive analysis may be necessary when the aroma of fermented ciders from dessert apples is unknown or when assessing novel processing practices.[Citation24] Untargeted metabolomics-based analyses also allow for identifying more of the volatiles that contribute to a cider’s overall character, including low-odor activity compounds that may be overlooked with targeted analysis. This is critical, as increasing research demonstrates that the overall aroma profile has many synergistic interactions rather than a single volatile being perceived in isolation.[Citation20,Citation25] An alternate model using the odor activity values (OAVs) of volatile compounds reflects their importance in contributing aroma notes to the overall aroma of the sample. OAV is calculated by comparing the concentrations in the sample with their perception threshold.[Citation26] While the OAV model has limitations, it can be a powerful tool in identifying odor-active compounds and prioritizing their impact on aromas by using it in conjunction with different techniques (such as GC-O). The usefulness of OAV can be further expanded in understanding complex aroma mixtures via GC-O coupled aroma extract dilution analysis (AEDA) and aroma recombination and omission experiments.[Citation27,Citation28] However, untargeted metabolomics analysis can compensate for the deficit of OAVs in understanding aroma by broadly examining all features and then highlighting those with greater potential in bioactivity.
Due to the difficulty in growing heirloom apples and the scarcity of information on fermented cider processed from dessert apples, eleven commonly grown cultivars were used for this investigation. This study aimed to elucidate the volatile profile of ciders processed from different cultivars of dessert apple using headspace solid-phase microextraction gas chromatography-mass spectrometry (HS-SPME-GC-MS) and to determine if extended skin maceration during processing increased the aroma in the final products.
Experimental
Chemicals
All aroma standards were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.) at >98.8% purity. A C7-C30 hydrocarbon mixture, used to determine Kovat’s retention indices, was obtained from Sigma-Aldrich. Sodium chloride and L-malic acid (99%) were purchased from Fisher Chemicals (Fair Lawn, NJ, U.S.A.). Ultrapure water (Type 1 water) was prepared using the ELGA Lab Water PURELAB Classic (High Wycombe, U.K.).
Preparation of ciders
Five (‘Galaxy Gala’ (G), ‘Granny Smith’ (GS), ‘UltraRed Jonathan’ (J), ‘Starkspur Supreme Red Delicious’ (RD) and ‘Winesap’ (W)) and eleven (aforementioned plus ‘Arkansas Black’ (AB), ‘Braeburn’ (B), ‘Yataka Fuji’ (F), ‘Golden Supreme Golden Delicious’ (GD), ‘Jonagold’ (JG), and ‘Pink Lady’ (PL)) common dessert apple cultivars, grown in Missouri (MO) were fermented in 2018 and 2019, respectively. Missouri apples were sourced and harvested at commercial ripeness for the cultivar from the Horticulture & Agroforestry Research Farm in New Franklin, MO (39°00’34.81” N latitude, 92°44’10.79” longitude) and a commercial orchard in Waverly, MO less than 80 km away (39°12’17.4” N latitude, 93°31’46.84” W longitude). The soil type at both locations was a Menfro silt loam (fine-silty, mixed, superactive, mesic typic hapludalfs) with pH 6.5. Trees were fertilized and drip-irrigated with pests controlled following local recommendations.[Citation29]
Following harvest, the fruit was cold stored at 10 °C for no more than three weeks until processing on 23 Oct. for early cultivars, and 14 Nov. for late cultivars. Pre-fermentation analysis was conducted on ten randomly selected apples or juice produced from approximately 80 kg of fruit for each cultivar. Flesh firmness (F.F. in N) was evaluated with a portable penetrometer (EFFEGI, FT 327, Italy) with an 8-mm tip. After removing small skin sections, firmness measurements were obtained from two opposite sides of each fruit. The pH of the juice samples was measured with a calibrated temperature-compensating pH probe and Orion 3-star meter (Thermo Scientific, Fort Collins, CO). Aliquots (2 ml) of juice were diluted to 50 ml with degassed, deionized water, and titratable acidity as malic acid (g/L) was measured using a Compact Titrator (Model G20, Mettler-Toledo, Schwerzenbach, Switzerland) to an endpoint of 8.2 pH, with 0.1 N sodium hydroxide (NaOH) solution. Soluble solids percentage was determined using a temperature-compensating digital refractometer (Reichert Technologies, Munich, Germany).
Fermentation conditions
A minimum of 80 kg of fruit was ground for each cultivar and divided into half. The ground fruit was either then directly added to 15 L plastic fermenters for the maceration treatment (treatment “M”) or pressed using a 20 L hydraulic bladder press in multiple batches and placed into similar 15 L fermenting vessels (treatment “C”). All fermenters included an airlock and were kept closed (except for when punch downs were conducted). Each cultivar produced six separate fermentations per year with an N = 3 for both treatments’ “C” and “M”. All fermentations were inoculated with DV-10 yeast (Scott Laboratories, Petaluma, CA) rehydrated with GoFerm™ (Scott Laboratories, Petaluma, CA) as per the manufacturer’s instructions with yeast inoculation and Goferm™ addition at 0.2 g/L and 0.3 g/L respectively. This addition equates to 10 ppm of yeast available nitrogen. All fermentations were conducted in a cold room held at 15 °C, until ciders reached dryness (less than 1 g/L residual sugar) as determined by AimTab™ (Germaine Laboratories, Inc., San Antonio, Texas). During fermentation, treatment “M” had the cap formed by the pomace separating from the cider and was reintegrated manually twice a day. Once treatment “M” ciders obtained dryness, they were pressed separately and racked into a 10 L glad fermentation vessel. Similarly, treatment “C” was also racked into a 10 L fermentation vessel with no headspace following primary fermentation. All ciders were stored for a minimum of 1-month, racked again, then bottled 1-week later in 350 ml crown top glass bottles, and kept at 5 °C until analysis.
Analysis of volatile compounds
Volatile analysis methodology and untargeted workflow were based on the volatile profiles of each cider sample from 2018 and 2019. Compounds, including esters, alcohols, acids, terpenes, ketones, and phenols, were extracted and concentrated in analytical triplicate by automated headspace solid-phase micro extraction and analyzed by GC-MS (HS-SPME-GC-MS). The cider samples (5 mL) were mixed with 100 µL of 10 ng/mL 4-methyl-2-pentanol for 2018 cider samples and 2 g NaCl and 20 µL of 12.5 ng/mL 2-octanol for 2019 cider samples (both internal standards dissolved in methanol) in a 20 mL vial tightly capped with a polytetrafluoroethylene-silicon septum. A 50/30 μm DVB/C-WR/PDMS SPME fiber was used for extraction (Supelco, Bellefonte, PA). The samples were preincubated at 40 °C with the fiber and then exposed to 40 °C for 30 min in the headspace above the sample before GC-MS analysis. All samples were agitated at 500 rpm during extraction. Then the fiber was desorbed in the inlet at 260 °C for 0.75 min in splitless mode (inlet glass liner/SPME direct, 0.75, I.D., Supelco), after which the split flow was turned on (15 mL/min) for the remainder of the GC-MS run. No carryover was observed between samples. To monitor potential carryover, a blank was run after every five samples. Two separate Agilent 7890 A gas chromatographs with Agilent 7000 triple quad detectors (Agilent Technologies, Forest Hill, Vic., Australia) were used to analyze the volatile compounds. A DB-WAXetr column (30 m x 0.25 mm ID., 0.25 μm film thickness; Agilent Santa Clara, CA, U.S.A.) and helium carrier gas (flow rate: 2 mL/min) were used for all analyses. The GC oven program was as follows: initial temperature of 35 °C for 5 min increased to 240 °C at 6 °C/min, which was held for 10 min. The source temperature, MS1 quadrupole, and MS2 quadrupole temperatures were set to 230 °C, 150 °C, and 150 °C, respectively. The electron ionization source operated at 70 eV. In the collision cell, the He quench gas was set at 2.25 mL/min, and nitrogen (99.999% purity, Praxair) at a 1.5 mL/min flow rate was used as collision gas. EI spectra were collected in scan mode from 35 to 350 amu, with 7.5 scans per second. Multiple reaction monitoring (MRM) was also used for the IS 2-Octanol due to interference with other cider volatiles in scan mode, MRM 97 m/z->55 m/z. Data acquisition and qualitative analyses were performed using the MassHunter Workstation software version B.07.00 (Agilent Technologies).
Data processing using untargeted metabolomics analysis
An untargeted metabolomics workflow was applied to find subtle differences in volatiles within cultivars and skin maceration.[Citation24] Data processing transforms were conducted using msconvert tool from Proteowizard. The raw chromatographic D. data files were converted into the mzML format that is useable for further analysis, which includes removing noise, feature detection, alignment, retention time correction, and normalization from each raw data file by using XCMS online, available at https://xcmsonline.scripps.edu/. The following uni/multivariate analysis based on the detected features was conducted by MetaboAnalyst (http://www.metaboanalyst.ca, McGill University, Montreal, QC, Canada).
Identification and confirmation of the compounds
After the identification of significant features using ANOVA (p < 0.05) and principal component analysis (PCA) loadings (between −0.01 and 0.01), the significant features were grouped based on their retention time. The compounds represented by the features were tentatively identified using the NIST MS Search v2.2, NIST 14 Mass Spectral Library database (Scientific Instrument Services, Ringoes, NJ, U.S.A.) by matching the mass spectral data with that of the compound. Only the compounds with a high match score (over 700) to the NIST database were considered. Additionally, linear retention indices (RI) were calculated using Kovats’ equation from a sequence of linear hydrocarbons from C7 to C30 to verify the NIST matches to that of the literature. Thus, two-step identification was made for the volatile compounds as possible matches were first identified by comparison of the mass spectral data within the NIST library and then verified as a valid prospect based on RI data. The confirmation and quantitation of volatile compounds were achieved using calibration curves for each standard at seven different concentration levels in cases where standards were available.
For the 2019 dataset, each standard was dissolved in HPLC-grade methanol and then was diluted with 5% (V/V) model cider (5% EtOH, 5 g/L malic acid, adjusted to pH 3.5 with 5 N NaOH) to generate the stock standard solution. The stock standard solution was then diluted into ten successive levels using the synthetic matrix (5% (V/V) model cider). Afterward, these standard solutions were extracted using the same volatile extraction procedure as the cider samples, and the analysis of the standards followed the same GC analytical method. The standard curve was integrated using the peak area ratio of the external volatile standard to the internal standard versus the concentration of the external standard. Semi-quantification of volatile compounds was achieved using the corresponding standard and applied for the following analysis. The semi-quantitative analyses were completed using MassHunter Quantitative Analysis software version B.07.01 (Agilent Technologies). Two separate instruments were used due to equipment availability, but all analysis parameters were the same. This shift meant that compounds were only presented as relative intensity in the 2018 dataset. However, more rigorous quantitative data was collected in 2019 ( and ) as the calibrations created would not be appropriate for the previous year’s GC-MS run. In the 2018 dataset, the peak area ratios of identified volatile compounds to internal standard were used to represent the relative abundance of volatile compounds to identify differences between cider samples (Tables S2 and S3).[Citation30] Given that 2019 was a more robust data set, results and discussions were initially based on 2019 findings, with 2018 used to validate and further understand trends.
Table 1. The contents of volatiles (ng/mL) with cultivars x treatments interaction in year 2019 (n = 3).
Table 2. The contents of volatiles (ng/mL) without cultivars x treatments interaction in year 2019 (n = 3).
Table 3. The profile of apple fruits and musts obtained from different apple cultivars at different fermenting years.
Odor activity value (OAV)
The odor thresholds used are presented in Table S1. A volatile compound OAV higher than one indicates that its aroma features likely contribute to the overall aroma of the sample, and higher values generally imply a compound has more influence than a compound with a lower OAV value.
Tannin measurement
Tannins were measured using the Adams-Harbertson (A-H) assay described by Harbertson et al.[Citation31] However, data are not presented as all measurements were below the limit of detection.
Statistical analyses
After the semi-quantification of the identified compounds from features, a two-way analysis of variance (Two-way ANOVA) was first performed to assess the interaction effect between cultivars and treatments (Table S1) in years 2018 and 2019. The volatiles with interaction effect were furthered analyzed by one-way ANOVA and Tukey’s post hoc test at α = 0.05 crossing over all the combinations of cultivars and treatments (Table S2 for 2018 and for 2019), while those without interaction effect were analyzed by one-way ANOVA and Tukey’s post hoc test crossing over cultivars and treatments, separately (Table S3 for 2018 and for 2019). Statistical univariate analyses here were performed by using R software 4.2.1; Boston, MA, U.S.A. Partial least squares regression discrimination analysis (PLS-DA) was applied for standardized data to study the differences and classifications among the apple cultivars (n = 5 in the 2018 dataset, n = 11 in the 2019 dataset) and treatments (n = 2 for both 2018 and 2019) using the volatile variables as X-data and aforementioned classes as Y-data. Full cross-validation was used to estimate the number of factors for a statistically reliable model. Data analysis for each year was conducted separately to avoid the instrumental effect and focus on cultivars and treatment variation.
Results and discussion
Chemical profile of dessert apple cultivars
All fruit was harvested (either commercially or from research orchards) at a point that met USDA maturity standards for a given cultivar (USDA, Apple Inspection Instructions).[Citation55] However, as maturity expectations are very cultivar dependent, this means that certain cultivars are inherently harvested with varying physical and chemical properties. To minimize ripening differences, apples were processed and fermented in two batches each year, grouped into early and late harvested cultivars. Ensuring no fruit was in cold storage longer than 3 wk. Prior to processing cider, the ripeness status of the dessert apples was characterized. Five and eleven cultivars of dessert apples were evaluated in 2018 and 2019, respectively, with the original five cultivars assessed again in 2019. Using the Long Ashton Research Station system, typically applied to heirloom cider apples, dessert apples were classified as "sweets" or "sharps" based on malic acid concentration (g/L).[Citation32] Given their low tannin concentrations (each < 2.0 g/L, based on the A-H assay, data not shown), none fell into the "bitters" category. Varied firmness, soluble solids (°Brix), titratable acidity (TA), and pH were observed among cultivars in both seasons (). Despite attempts to control for ripeness, there was great variability in some cultivars between 2018 and 2019 (e.g. ‘Galaxy Gala’ and ‘Winesap’). While some of this variability is likely due to seasonal differences and therefore is an accurate representation of the variability of that cultivar, differences in harvest maturity and post-harvest ripening both between cultivars and years should be considered when interpreting findings below. Due to the inherent phenotypic differences in apple cultivars, including sugar content when ripe, and harvest date, it is impossible to compare cultivars without some confounding factors.[Citation17] Additional research into individual cultivars may be needed to parse out the degree to which cultivar differences can be mediated by other factors in the field or cidery.
During ripening, various changes occur related to primary and secondary metabolites, including changes in sugar content and increase or decrease in volatile compounds. It has been observed previously that firmness generally decreases while sugar content increases during maturation and ripening to a point. Sugar then decreases in overripe fruit due to it being consumed during respiration.[Citation33,Citation34] Ciders made from over-mature apples slowly lose some acidity and “juiciness” causing a flatter flavor.[Citation35] Increased ripening can also be advantageous for cider production as softer fruit will yield more juice, and higher starch conversion will increase sugar and, ultimately alcohol content. In our study, despite trying to harvest at the same maturity each year and among cultivars, extremely low firmness in ‘Galaxy Gala’ was observed in 2018, likely due to overripening. The TA for ‘Granny Smith’ was lower in 2019 compared to 2018, which was also potentially due to the impact of different degrees of ripening in each year (). While it’s common to cold store apples intentionally in cider production, none of our fruit was stored for more than three weeks to minimize cultivar differences from post-harvest ripening. Although maturity measurements were not recorded immediately after harvesting, differences in ripeness were likely due to harvest timing rather than storage.
The below investigation of volatiles should be considered in light of ripeness metrics, as sugar content, titratable acidity, pH, and secondary metabolites are impacted by the degree of fruit ripening.[Citation36]
Untargeted metabolomics
As only a limited amount of previous investigation has been done into the aroma chemistry of ciders made from dessert apples, an untargeted analysis was undertaken to allow for a comprehensive investigation of volatile chemistry. Untargeted analysis of GC-MS data is a powerful tool for identifying compounds of interest in poorly studied systems.[Citation24] Rather than focusing on a limited selection of identified compounds, individual features are extracted for the screening of all metabolites detected by MS. Each feature denotes an intensity for a m/z for a given time, and one compound may contribute multiple features (e.g., the aroma compound eugenol will elute at a given time, but create multiple ions with m/z intensities). A total of 1482 and 1226 features were identified in the GC-MS data of cider samples from 2018 and 2019, respectively. Only features significantly impacted by cultivar or treatment, as indicated by one-way ANOVA, were included for further analysis. There were 1340 and 1159 significant features identified in 2018 and 2019, respectively, impacted by both cultivar and treatment effects (p < 0.05).
The number of significant features found in this study is similar to other untargeted metabolomics research assessing treatment and cultivar differences in grapevines,[Citation37] wine,[Citation24] rice,[Citation38] and oolong tea.[Citation23] These results indicate that the initial untargeted analysis of cider samples by GC-MS in the present study was effective in identifying a large number of volatile differences for further investigation. In studies that start with targeted analysis, far fewer potential chemical differences are evaluated, often focusing on less than ten compounds of interest.[Citation21] Whereas in untargeted screening, one must compromise between optimization for specific compounds and inclusive detection to include a more diverse range of potential chemicals. While both approaches have their advantages and disadvantages, untargeted analysis was chosen, due to the limited knowledge about the volatiles that are important in post-fermentation for the cultivars studied. This was done to mitigate the risk of overlooking crucial metabolites that define the variability across cultivars and treatments.
In our study, all the volatile metabolites and features were exclusively assessed in the ciders, as opposed to the apples or unfermented juice. This choice was made because many primary apple aromas persist in cider, and alcoholic fermentation also yields new compounds that arise from the yeast metabolism of apple precursors,[Citation14] as are classified in .
Following feature detection, principal components analysis (PCA) was used to assess the ability of these features to differentiate cultivars in traditionally produced cider as well as cider made with maceration during fermentation. Analyses of traditional () and macerated ciders () were conducted separately in 2018 to assess what degree clear juice or pomace-related constituents (e.g., peels and seeds) from different cultivars impact cider volatiles. This analysis was repeated in 2019 and presented in . In the interest of brevity, we have focused on 2019 untargeted data, as it included a greater number of cultivars.
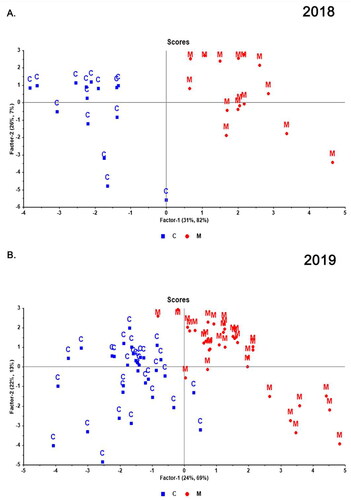
Figure 2. Scores plot from PCA of apple cider volatile features separated by cultivars for 2018 and 2019. A and B are the plots for ciders made from pressed juice (treatment ‘C’) in 2018 and 2019, (i.e., traditionally produced cider). C and D are the plots for ciders made with maceration of apple pomace (treatment ‘M’) in 2018 and 2019. PCA was performed using log-transformed and auto-scaled significant features. Cultivar abbreviations refer to AB, ‘Arkansas Black’, B, ‘Braeburn’, F, ‘Yataka Fuji’, G, ‘Galaxy Gala’, GD, ‘Golden Supreme Golden Delicious’, GS, ‘Granny Smith’, J, ‘UltraRed Jonathan’, JG, ‘Jonagold’, PL, ‘Pink Lady’, RD, ‘Starkspur Supreme Red Delicious’, W, ‘Winesap’.
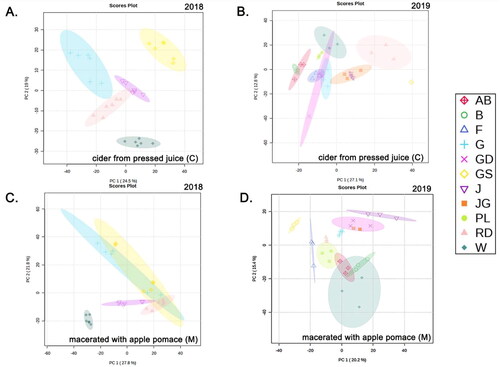
In 2019, clear separation was observed in both the traditionally made ciders () and ciders macerated with apple pomace (). The model was able to explain 27.1% and 12.8% of the total variance by PC1 and PC2, respectively, in cultivars for traditionally produced ciders (treatment ‘C’) in 2019 (). When modeling with only macerated (treatment ‘M’) samples, the two components cumulatively accounted for 35.6% of the total variance (as compared to 39.9% for traditionally pressed ciders), with PC 1 and PC 2 explaining 20.2% and 15.4% of the variance, respectively (). Both of the explained variances are in the acceptable range when compared to previous similar works.[Citation23,Citation24,Citation37,Citation38] Although over half of the variance remained undefined in PC1 and PC2, the replicated cultivar samples clustered together despite randomized data collection and replicated fermentation. This demonstrated that the MS analysis had captured features driving strong cultivar differences, though explanation of those differences required further data analysis to identify the compounds driving the separation.
The PCA revealed the cultivars replicated in 2018 and 2019 clearly separated based on their volatile features despite differences in ripeness in the two years (). For instance, Granny Smith was heavily separated from all cultivars on PC1 for both years with Winesap separating along PC2 in both years. After introducing the other six cultivars in the 2019 dataset, some cultivars tended to overlap with each other. ‘Arkansas Black’ (AB) and ‘Braeburn’ (B) or ‘Winesap’ (W) and ‘Pink Lady’ (PL) overlapped, indicating that these cultivars tend to share similar volatiles and volatile concentrations. Of note, ‘Galaxy Gala’ (G) and ‘Yataka Fuji’ (F) were both categorized in the sweets apple category () clustered together after cider processing. Conversely, ‘Pink Lady,’ classified as sharp with the highest malic acid concentration, separated from ‘Starkspur Supreme Red Delicious’ (RD) cider, categorized as sweet. This aligns with previous research, indicating that volatile compounds in cider closely relate to apple sweet or sharp categorization.[Citation39] Shared precursor chemistry, involving apple metabolites like organic acids, phenolics, and amino acids,[Citation14,Citation40,Citation41] may explain why cultivars falling into the same category in the Long Ashton Research Station system exhibit similar cider volatiles. Further research is needed to define what features and volatiles might be influenced by the degree of ripeness, namely sugar content and organic acids which could further drive or minimize the cultivar separations we observed. Additionally, it should be investigated if changing must chemistry through addition of sugar, or acid, would ultimately shift some of the fermentation mediated volatiles closer to that of another cultivar.
Although there was good separation in treatment ‘C’ in 2018 (), all cultivars overlapped with the exception of ‘Winesap’ for treatment ‘M’ (). This shift from clear separation of features to overlap, indicates maceration induces homogenization of volatiles potentially due to apple pomace contributing more common volatiles and precursors than free juice regardless of the cultivar.[Citation19,Citation42] This homogeneity among ‘M’ ciders was not observed in for all 2019 cultivars (), which may be due to the inclusion of more diverse cultivars, which enhanced volatile diversity and separation.
Contribution of cultivar to the volatile compounds in apple cider
Apple cider aroma compounds can be grouped into chemical classes such as alcohols, esters, fatty acids, and carbonyls.[Citation21,Citation43,Citation44] We were able to identify 19 and 22 aroma-active compounds in 2018 and 2019, respectively, which had contributed to the features pushing separation by cultivar and treatment in the untargeted analysis. The following identification was accomplished by comparison of retention time and spectra to authentic standards, following tentative identification by NIST spectral database. This intensive process for identification was undertaken, as many of the compounds tentatively identified by spectra alone share a large portion of their MS spectra with other compounds in their class. This time consuming and expensive identification procedure is ultimately necessary if untargeted results are to be used to understand complex biological systems or relate to published aroma thresholds.
The compounds identified were comprised of nine esters, six higher alcohols, two fatty acids, two monoterpenes, two volatile phenols, and one ketone. Many of the same identified aroma chemicals existed in samples from both years. Ethyl 2-methylbutyrate, diethyl succinate, and 4-ethyl-2-methoxyphenol were only found in 2019 samples, which may be due to true seasonal differences. Some of the compounds were found to have significant cultivar by treatment interactions ( and S2), while the differences in others were impacted by either cultivar or treatment ( and S3). Due to the instrumental availability, all the compounds identified in 2018 were presented as relative responses, while all the compounds were quantified in 2019 with calibration curves made from authentic standards. We observed wide diversity in the concentrations of some compounds, such as citronellol, with a relative response of 2.6-10.1 in 2018 (Table S2), and ethyl 2-methylbutyrate which ranged from 8.8 ng/mL-379.8 ng/mL in 2019 (). While great variation in concentration existed between cultivars, all compounds identified have been previously found in ciders.
Volatile compounds, such as ethyl 2-methyl butanoate (E5), ethyl hexanoate (E1), octanoic acid (A1), and eugenol (V2) were found in high concentrations in our study. These compounds are also among the most important volatiles in ciders processed from heirloom apples,[Citation43,Citation44] which were further compared in their concentration after log transformation shown in . We found the concentrations of these compounds in ciders from dessert apples higher than those previously reported for heirloom apple ciders. As an example, in the ciders produced using traditional methods, the concentration of 1-octanol (H3) log transformed concentration from 0.9 to 1.6 ng/ml (), which was notably higher than the 0.0-0.6 ng/ml log range observed by others in heirloom apple ciders. Similarly, ethyl hexanoate (E1) showed concentrations log transformed range from 2.3 to 2.8 ng/ml (), surpassing the range of 1.1-2.4 ng/ml found in heirloom apple ciders.[Citation44] These consistent differences in some fermentation mediated aroma compounds suggest that there is something inherently different between the chemistry of the dessert apples we tested and many heirloom cultivars. As our sugar and acid ranges fell within what would be considered typical for apples destined for cider production, some other factors such as amino acids, polysaccharides or other precursors must be ones driving the difference. Regardless of the mechanism, this further bolsters the observation that there may be value for dessert apples in blending, as dessert apples offer different chemistry to that observed in heirloom cultivars.[Citation11]
Figure 3. Comparison of cider volatile concentrations observed in in ciders produced from dessert apples in 2019 in our work compared to the concentration observed in heirloom apples by others.[Citation43,Citation44] To allow factors to be plotted in a manner that allows comparison, concentrations have been log transformed. The volatile compounds and compound classes correspond with our data presented in and . Abbreviations of compounds presented are as follows: E5, ethyl 2-methylbutanoate, E1, ethyl hexanoate, E6, hexyl acetate, H1, 2-Phenylethanol, H2, 1-hexanol, H3, 1-octanol, V2, eugenol, and V1, 4-ethyl-2-methoxyphenol. Data from 2018 was not included as it was not quantitative.
![Figure 3. Comparison of cider volatile concentrations observed in in ciders produced from dessert apples in 2019 in our work compared to the concentration observed in heirloom apples by others.[Citation43,Citation44] To allow factors to be plotted in a manner that allows comparison, concentrations have been log transformed. The volatile compounds and compound classes correspond with our data presented in Tables 1 and 2. Abbreviations of compounds presented are as follows: E5, ethyl 2-methylbutanoate, E1, ethyl hexanoate, E6, hexyl acetate, H1, 2-Phenylethanol, H2, 1-hexanol, H3, 1-octanol, V2, eugenol, and V1, 4-ethyl-2-methoxyphenol. Data from 2018 was not included as it was not quantitative.](/cms/asset/a1355e27-5a5e-4763-a048-8c8c1485b3ff/ujbc_a_2319931_f0003_c.jpg)
There are three general paths for flavor formation in cider (). When apple juice is fermented into cider, yeast converts sugars into ethanol, carbon dioxide and primary yeast metabolites that are flavor active (source A). This will occur regardless of fruit type, though the ultimate concentrations of metabolites produced are impacted by the concentrations available for fermentation. Second, aromas are created from yeast by utilizing aroma-precursors in the apple, or the chemical compounds of the specific juice (source B). Compounds from this source are considered as yeast secondary metabolites, such as higher alcohols, volatile phenols, and certain esters. They are created during fermentation but highly dependent on the substrate the yeast is utilizing. The third path of the flavors in cider are directly contributed by the apple that were used to produce ciders and is therefore highly cultivar dependent (source C). This group includes some of the esters and fatty acids detected in this study.
Esters are an important aroma class in ciders, mostly produced through esterification of various precursors as yeast secondary metabolites (source B of ). The presence of amino acids is important in the esterification process during fermentation, as they serve as intermediates in the pathway, particularly when higher alcohols and ethanol are involved.[Citation40] While most esters come from fermentation,[Citation45] compounds in the apple and apple juice, such as ethyl 2-methylbutyrate (E5), ethyl hexanoate (E1), and hexyl acetate (E6) originally produced by apples and remain in the final cider products along with apple derived odor active fatty acids, such as octanoic acid (A1) and hexanoic acid (A2) found in this study,[Citation45] which shown in source C of .
Trained panelists have previously identified all identified esters in this work to have odor descriptors such as sweet, fruity, and floral (Table S1). These compounds have also been found to have a favorable synergistic impact on aroma.[Citation25] While esters from fermentation (source B) are generally more pronounced, ethyl 2-methylbutyrate and hexyl acetate derived from apple aroma (source C) had an OAV exceeding 1, indicating at the concentrations we observed they should contribute cider aroma to a greater or lesser extent depending on the cultivar.
Esters have been found to be impacted by the degree of ripeness of the fruit with fruit harvest from senescent trees having up to 52% higher concentration of esters than those deemed unripe.[Citation42] However, no difference was observed under milder variations in ripeness.[Citation42] As there is some evidence ripeness can impact ester concentration, we investigated the relationship between ripeness parameters across cultivars and individual/total esters. For our 2019 data neither sugar content or titratable acidity was able to explain much of the variance in esters with the correlation between mean °Brix and total concentration of esters of R2=0.1944, and mean TA and total concentration of esters R2=0.0055 (data not shown). Correlations between induvial esters and basic must chemistry were similarly poor suggesting at least across cultivars, the influence of cultivar was greater than the degree of ripeness on ester concentration. Other factors such as must amino content, which was not measured in this study, may also account for some of the differences in esters. The impact on certain amino acids is influenced by factors such as cultivar and tree nutrition.
Quantitatively, higher alcohols are the most abundant group of volatile compounds produced by yeast during alcohol fermentation through the catabolism of amino acids in apples via the Ehrlich pathway, displayed in source B of .[Citation46] Ciders in this study were rich in phenylethyl alcohol (H1, floral, and rose) and 1-hexanol (H2, green, and pungent), similar to previous findings on apple cider.[Citation44]
When comparing the total sum of relative responses between traditional ciders made from pressed juice in the 2018 dataset, it was observed that only the ‘Galaxy Gala’ cider was significantly higher in identified volatile compounds. In contrast, there was no statistically significant difference among the other ciders in terms of their total volatile compound profiles (Table S4 and Figure S1). It was observed that ‘Galaxy Gala’ cider was not only high in total abundance of volatile compounds but had the highest relative response among all the identified aroma classes as well, especially in higher alcohols and esters (Table S4). In PLS-DA analysis, ‘Galaxy Gala’ exhibited significant variation among five cultivars, with a strong positive correlation to compounds mainly among esters and high alcohols, but also included other classes of compounds, such as 1-butanol (H6, which has been described as fuel, oil, sweet, balsam, and whiskey), ethyl octanoate (E7, fruity, wine, waxy, sweet, apricot, banana, brandy, and pear), eugenol (V2, sweet, spicy, clove and woody), ethyl decanoate (E2, sweet, waxy, fruity, apple, grape, oily, and brandy) and ß-damascenone (K1, woody, sweet, fruity, earthy, green, and floral) ().
Figure 4. PLS-DA models for cider samples in 2018/2019 classified using the specific volatile compounds as X data (n = 19/22) and cultivars as Y-data (n = 5/11) measured by GC-MS. The volatile compounds are measured as relative response in 2018 (plots A. and C.) and concentrations in 2019 (plots B and D). Cultivar abbreviations refer to AB, ‘Arkansas Black’, B, ‘Braeburn’, F, ‘Yataka Fuji’, G, ‘Galaxy Gala’, GD, ‘Golden Supreme Golden Delicious’, GS, ‘Granny Smith’, J, ‘UltraRed Jonathan’, JG, ‘Jonagold’, PL, ‘Pink Lady’, RD, ‘Starkspur Supreme Red Delicious’, W, ‘Winesap’. Abbreviations of volatiles in the loading plots refer to Table S1 (e.g., E1, ethyl hexanoate, H1, phenylethyl alcohol, A1, octanoic acid, T1, linalool, V1, 4-ethyl-2-methoxyphenol, K1, ß-damascenone).
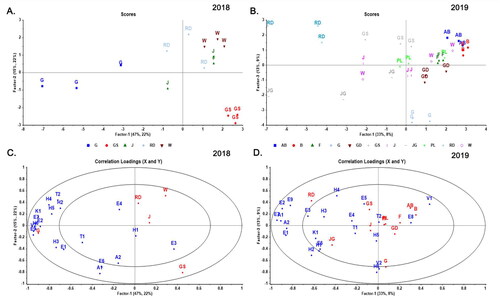
The key differentiating esters between ‘Galaxy Gala’ cider and other samples in this study primarily originate from yeast metabolites (Source B of ) produced during fermentation rather than being directly sourced from the original apple fruits (Source C). Nonetheless, these yeast intermediate esters still exhibit a strong connection to the fundamental composition of the cultivar fermented. Previously ‘Gala’ cider samples have been found to contain ethyl octanoate and ethyl decanoate and were at higher concentration when compared to ‘Royal Gala’.[Citation41] Since is a sport ‘Royal Gala’ of Gala’ it is reasonable to infer that the variations in amino acid content are likely the dominant factors responsible for the differences in the abundance of volatile compounds in the ciders. ‘Gala’ is one of the most widely grown dessert apple cultivars with basic chemistry suitable for making fermented cider.[Citation10] This study expands our understanding of ‘Gala’ cider aromatics, suggesting potential benefits in blending ciders with intense aromatics to enhance those with deficiencies.
‘Granny Smith’ was observed to have the second most variation (R2 = 0.962; validated R2 = 0.874). It was located in the bottom right of the plot and is separated from the rest of the samples in both factors with a negative correlation to volatiles, such as ß-damascenone (K1) and ethyl octanoate (E7). ‘Granny Smith’ has been previously proposed as a desirable dessert apple for making sharp/sharp-sweet ciders with tart and acidic flavor. However, cider made of ‘Granny Smith’ may be lacking in fruity and floral aroma due to less sugar converting to the fermentation products of alcohol and carbon dioxide, thus creating less abundance in the final volatiles.[Citation47] ‘Starkspur Supreme Red Delicious, and ‘UltraRed Jonathan’ were not equally well- classified in the model. Also, ‘Winesap’ lay opposite to most of the aroma compounds in the first quadrant and had a significantly lower total sum of relative response compared to ‘Galaxy Gala’ shown in Figure S1.
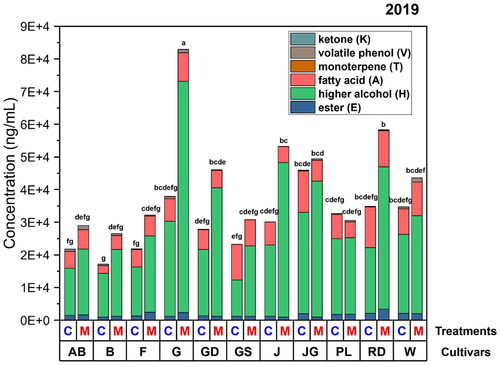
In the 2019 dataset, ‘Jonagold’ cider had the highest total sum concentration among all cultivars that are made from traditional pressed juice (). It was significantly higher in all classes of compounds except volatile phenols (Table S5). It was observed in previous research that ‘Jonagold’ apples have a higher content of amino acids than apples of ‘Yataka Fuji’, ‘Granny Smith’ and ‘Golden Supreme Golden Delicious’, which could explain the volatile abundance in cider products.[Citation48] In contrast, ‘Galaxy Gala’ produced the highest aroma abundant ciders after the maceration, with a dramatic increase in the content of higher alcohol (). The higher alcohols were usually considered as a yeast intermediate class of compounds (source B of ). However, it was shown in previous research that aromatic alcohols can also exist in the conjugated form bound to mono/disaccharides.[Citation49] It is likely that the significant increase in aroma compounds was due to the ‘Galaxy Gala’ apples having higher concentrations of volatile precursors in pomace than other cultivars.[Citation50]
Figure 5. Total volatile concentrations segmented into compound classes (K, ketone, V, volatile phenol, T, monoterpenes, A, fatty acids, H, higher alcohols, and E, esters) for two treatments (shown in top line of x axis) and eleven cultivars (in bottom line of x axis) in 2019. Treatment abbreviation ‘C’ denotes traditionally made ciders from pressed juice and ‘M’ denotes ciders that included maceration of apple pomace. Cultivar abbreviations refer to AB, ‘Arkansas Black’, B, ‘Braeburn’, F, ‘Yataka Fuji’, G, ‘Galaxy Gala’, GD, ‘Golden Supreme Golden Delicious’, GS, ‘Granny Smith’, J, ‘UltraRed Jonathan’, JG, ‘Jonagold’, PL, ‘Pink Lady’, RD, ‘Starkspur Supreme Red Delicious’, W, ‘Winesap’. Different letters above bars indicate significant differences at α = 0.05 by Tukey HSD compared with the sum of the concentration from each class of compounds measured. The significant difference (p < 0.05) between each compound class is included in the Table S5. Data from 2018 is presented in Figure S1.
In 2019, 11 cultivars were classified in PLS-DA with a model of seven validated factors (R2 = 0.582; validated R2 = 0.201). Notably, ‘Starkspur Supreme Red Delicious’, ‘Jonagold’, and ‘Galaxy Gala’ were the only three cultivars distinctly separated in the first and third factors of . ‘Starkspur Supreme Red Delicious’ was located on the left side of the plot and is correlated with generally perceived pleasant volatiles, such as methyl hexanoate (E3, ethereal, fruity, pineapple, apricot, strawberry, tropical, fruit, banana, and bacon) and 1-octanol (H3, citrus, floral, and fatty). ‘Jonagold’ located in the third quadrant, was the most volatile-abundant cultivar and is correlated with generally perceived pleasant volatiles, such as ß-damascenone (K1) and hexyl acetate (E6, fruity, green, apple, banana, and sweet). It was also highly correlated to phenylethyl alcohol (H1) and 1-hexanol (H2), which was consistent with previous research that ‘Jonagold’ has a high content of amino acids as the precursors.[Citation48] Arkansas Black’ and ‘Braeburn’ had similar volatile profiles as ciders (), which were abundant in volatile phenols (V1, 4-ethyl-2-methoxyphenol) and diethyl succinate (E8, mild, fruity, cooked, apple). Other cultivars were not modeled well by PLS-DA (), likely due to their relatively low volatile concentrations ().
Despite attempts to control for harvest maturity, variations between cultivars likely influenced the observed results.[Citation44] Additionally, seasonal variation and location can have an important impact on flavor development.[Citation51] ‘Granny Smith’ shown in and was generally among the cultivars with lowest total volatiles in 2018 and 2019. However in 2019, it did rank as having high concentrations of individual compounds such as ethyl 2-methylbutyrate (E5) and hexanoic acid (A2). This variation in individual aromas for GS may be due to the degree of ripeness as indicated by the relatively high titratable acidity in 2018 versus 2019 (). In a PLS-DA of quantified compounds ‘Galaxy Gala’ shown in is well separated from the rest of the cultivars. Driven by proximity to compounds, such as eugenol (V2) and 1-butanol (H6), similarly in 2018. However, it was negatively correlated to ethyl octanoate (E7), ß-damascenone (K1), and ethyl decanoate (E2), which were all abundant in ‘Galaxy Gala’ in 2018. Also, the 2018 ‘Galaxy Gala’ samples considered as overripe, had a high sugar content (), which could lead to a higher concentration of alcohol in fermented cider in that year compared with 2019. This result is consistent with the previous research by Venkatachalam et al.[Citation36] and also explains the difference in why cider samples from overripe fruit contained a higher level of aroma compounds than cider from ripe fruit.
Of note, ‘Winesap’ had the second-highest total concentration of volatiles in 2019, contrasting with its limited volatile abundance in 2018. This result may be attributed to the differences in ripeness as indicated by the firmness and °Brix values among the fruit sampled in 2018 and 2019 (), which in turn may be the result of environmental differences between seasons. Mechanical firmness and sugar levels are crucial in understanding post-fermentation volatiles, impacting yeast metabolism and final cider aroma.[Citation33,Citation34,Citation52] This complexity underscores the intricate interplay of fruit compositions, directly and indirectly influencing yeast metabolism, with primary apple aromas potentially carrying through to finished cider. Despite many fermentation pathways having been characterized, predicting the final volatile composition of a fermented product based on the initial ingredient remains impossible. Hence, relatively small differences in apple quality, as observed with ‘Winesap,’ can result in substantial post-fermentation variations.
Ultimately factors other than cultivars may have influenced our findings, but the large differences observed in various chemical classes between cultivars are far greater than what has been observed when studying other factors. With factors such as nutrition, amino acid content and ripeness causing at most a 50% shift in concentration,[Citation41] whereas with some compounds, such as ethyl decanoate or eugenol, we observed over a 10-fold difference between cultivars, indicating that cultivar can be a far stronger driver in cider aroma than other factors.
Contribution of skin maceration to the volatiles’ composition
Even in traditional (treatment ‘C’) cider fermentations, we observed a substantial correlation with the inherent attributes of the cultivar used. While we expected that increased cultivar influences may be introduced when the fruit was fermented on the skins and seeds (treatment ‘M’), in most cases this treatment tended to diminish the variation in aromas influenced by cultivar. The impact of the ‘C’ and ‘M’ treatments on the volatile composition is shown in . All sample analysis was carried out by PLS-DA using the same number of volatiles as X-data shown in (n = 19 in 2018; n = 22 in 2019) and two treatments (‘C’ and ‘M’ trials) as Y-data.
Figure 6. The scores plot of PLS-DA models for the impact of maceration during fermentation on cider volatile compound measured by GC-MS for all cultivars compared by treatment only in (A) 2018 and (B) 2019. ‘C’ denotes traditionally made ciders from pressed juice and ‘M’ denotes ciders that included maceration of apple pomace.
PLS-DA was conducted in 2018, with good separation with ‘C’ and ‘M’ samples displayed. The first factor explained 82% of the variance of the Y variables (). The skin maceration samples were also located in opposite quadrants to the pressed juice samples. The result was also confirmed by the univariate one-way ANOVA in Table S3 that all the compounds without the treatment and cultivars interaction in 2018 had a significant difference between the ‘C’ and ‘M’ treatments.
In 2019, two treatments were successfully classified in the model: ‘M’ with seven validated factors (R2 = 0.938; validated R2 = 0.886) and ‘C’ with seven validated factors (R2 = 0.938; validated R2 = 0.886). The separation can be displayed with the first two factors shown in . The ‘M’ treatment had a more dominating effect on increasing the OAV to > 1 than the impact of the cultivar (). For example, after applying apple pomace maceration, the concentrations of 1-hexanol increased in all cultivars, most notably in the ‘Granny Smith’ sample. Not all of the volatiles increased significantly, but some of the volatiles in the skin maceration treatment had OAVs > 1, while those in the no maceration treatment with pressed fresh juice remained below 1 (). Although the concentration of fatty acids was more dependent on the interaction impact than the treatments only, the OAV of hexanoic acid increased from ‘C’ to ‘M’ treatment in cultivars AB and G. In addition, the concentration of hexyl acetate was above the odor threshold after applying apple pomace maceration in ‘Yataka Fuji’ (). As Hexyl acetate is derived directly from apple (defined as source C of aromas in ), it would be expected to increase with additional pomace contact, as was observed.
Figure 7. The odor activity value (OAV) of a subset of volatile compounds and cultivars in 2019. A paired t-test was conducted for the OAVs within cultivars to assess the treatment effects of each compound. Different letters indicate significant differences at a level of 0.05 by Tukey HSD. The use of letters is omitted in cases where no significant differences were observed. Abbreviations of cultivars refer to AB ‘Arkansas Black’, B, ‘Braeburn’, GS, ‘Granny Smith’, G, ‘Galaxy Gala’, F, ‘Yataka Fuji’. ‘C’ denotes traditionally made ciders from pressed juice and ‘M’ denotes ciders that included maceration of apple pomace. “BLR” represents “below linear range” when concentration of the compound was below the linear range to calculate the OAV. The compounds above the red line have an OAV larger than 1. Data of 2018 was not available to calculate the OAV because data was presented as the relative response.
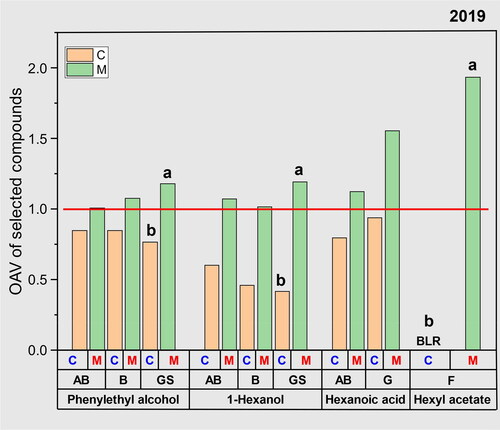
In red wine research, free and glycosidically bound volatile compounds influence the olfactory characteristics of fermented products. Moreover, it has been observed that an extended skin maceration time in wine positively impacts these compounds.[Citation53] Similar to grapes, apples were found to contain glycosidically bound volatiles, such as phenylethyl alcohol and ethyl 2-methylbutyrate,[Citation49,Citation54] which were also found in this study. We confirmed that this same principle in wine, when applied to cider making, can be of great value. Phenylethyl alcohol content increased in all cultivars with OAV > 1 after ‘M’ treatment, indicating a release of glycosidically bound compounds (). This treatment also significantly enhanced volatiles in cultivars with low volatile composition, such as ‘Yataka Fuji,’ leading to a 14-fold increase in concentration of ethyl 2-methylbutyrate (from 21.2 ng/mL to 299.0 ng/mL) (). This has the potential to elevate the fresh and fruity attributes of apple aroma in cider, positively impacting overall palatability.
Conclusion
Dessert apples produce ciders with varied volatile chemistry, with many of the compounds previously found in heirloom cider cultivars. Many compounds that defined the differences between cultivars were yeast-derived esters and higher alcohols. The significance of yeast-derived compounds in distinguishing between different cultivars highlights the importance of thoroughly characterizing ingredients intended for fermentation. Additionally, it illustrates that various non-volatile distinctions in fruit can have a direct impact on the ultimate volatile profile of the fermented products. Among the dessert apples evaluated, the ciders from ‘Galaxy Gala’, ‘Starkspur Supreme Red Delicious’, and ‘Jonagold’ had the most abundant aroma. Maceration did not introduce new aroma compounds but elevated the concentration of specific volatiles, in some cases surpassing published aroma detection thresholds. For example, the ethyl 2-methylbutyrate in the ‘Yataka Fuji’ samples increased 14-fold after apple pomace maceration. Generally, maceration increased the homogeneity between cultivars. While all aromas identified have been previously found in other ciders, some of the dessert cultivars produced concentrations orders of magnitude higher than what has been observed from heirloom cultivars. Thus, dessert apples may be valuable in blending, given their intense aroma. The higher intensity in volatile compounds, even those generally considered as pleasant, may not always be desirable, and as such, sensory evaluation of dessert cultivars and ciders produced with skin maceration would be a valuable next step. This work should be considered as a first step in characterizing the aromas that ciders from dessert apples may contain. Finally, further work related to processing, nutrient management and optimum ripeness is needed.
Author contributions (credit)
Yanxin Lin: conceptualization, formal analysis, investigation, writing (original draft, review, and editing), visualization.
Michele R. Warmund: funding acquisition, resources, writing (review and editing), supervision.
Misha T. Kwasniewski: conceptualization, resources, writing (review and editing), supervision, project administration, funding acquisition.
Supplementary Figures and Tables.docx
Download MS Word (91.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- FAOSTAT. “Value of Agricultural Production.”; 2021 March 18, 2021. https://www.fao.org/faostat/en/#data/QV.
- Horgan, F. G.; Launders, M.; Mundaca, E. A.; Crisol-Martínez, E. Effects of Intraspecific Competition and Larval Size on Bioconversion of Apple Pomace Inoculated with Black Soldier Fly. Agriculture 2023, 13(2), 452. DOI: 10.3390/agriculture13020452.
- U.S. Apple Crop Facts. 2022. https://pickyourown.org/USapplecrop.htm.
- Cairns, P.; Hamilton, L.; Racine, K.; Phetxumphou, K.; Ma, S.; Lahne, J.; Gallagher, D.; Huang, H.; Moore, A. N.; Stewart, A. C.; et al. Effects of Hydroxycinnamates and Exogenous Yeast Assimilable Nitrogen on Cider Aroma and Fermentation Performance. J. Am. Soc. Brew. Chem. 2022, 80(3), 236–247. DOI: 10.1080/03610470.2021.1968171.
- Kessinger, J.; Earnhart, G.; Hamilton, L.; Phetxumphou, K.; Neill, C.; Stewart, A. C.; Lahne, J. Exploring Perceptions and Categorization of Virginia Hard Ciders through the Application of Sorting Tasks. J. Am. Soc. Brew. Chem. 2021, 79(2), 187–200. DOI: 10.1080/03610470.2020.1843927.
- Leforestier, D.; Ravon, E.; Muranty, H.; Cornille, A.; Lemaire, C.; Giraud, T.; Durel, C.-E.; Branca, A. Genomic Basis of the Differences Between Cider and Dessert Apple Varieties. Evol. Appl. 2015, 8(7), 650–661. DOI: 10.1111/eva.12270.
- Bortolini, D. G.; Benvenutti, L.; Demiate, I. M.; Nogueira, A.; Alberti, A.; Zielinski, A. A. F. A New Approach to the Use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT 2020, 126, 109316. DOI: 10.1016/j.lwt.2020.109316.
- Vysini, E.; et al. ‘Sustainable Cider Apple Production’; 2012, p. 145.
- VanderWeide, J.; van Nocker, S.; Gottschalk, C. Meta-Analysis of Apple (Malus × domestica Borkh.) Fruit and Juice Quality Traits for Potential Use in Hard Cider Production. Plants. People. Planet. 2022, 4(5), 463–475. DOI: 10.1002/ppp3.10262.
- Soomro, T.; Watts, S.; Migicovsky, Z.; Myles, S. Cider and Dessert Apples: What Is the Difference? Plants. People. Planet. 2022, 4(6), 593–598. DOI: 10.1002/ppp3.10284.
- Cline, J. A.; Plotkowski, D.; Beneff, A. Juice Attributes of Ontario-Grown Culinary (Dessert) Apples for Cider. Can. J. Plant Sci. 2021, 101(4), 536–545. DOI: 10.1139/cjps-2020-0223.
- Littleson, B.; Chang, E.; Neill, C.; Phetxumphou, K.; Sandbrook, A.; Stewart, A.; Lahne, J. Sensory and Chemical Properties of Virginia Hard Cider: Effects of Apple Cultivar Selection and Fermentation Strategy. J. Am. Soc. Brew. Chem. 2023, 81(1), 141–154. DOI: 10.1080/03610470.2022.2057780.
- Schreier, P.; Jennings, W. G. Flavor Composition of Wines: A Review. CRC Crit. Rev. Food Sci. Nutr. 1979, 12(1), 59–111. DOI: 10.1080/10408397909527273.
- Ye, M.; Yue, T.; Yuan, Y. Changes in the Profile of Volatile Compounds and Amino Acids During Cider Fermentation Using Dessert Variety of Apples. Eur. Food Res. Technol. 2014, 239(1), 67–77. DOI: 10.1007/s00217-014-2204-1.
- Corollaro, M. L.; Endrizzi, I.; Bertolini, A.; Aprea, E.; Demattè, M. L.; Costa, F.; Biasioli, F.; Gasperi, F. Sensory Profiling of Apple: Methodological Aspects, Cultivar Characterisation and Postharvest Changes. Postharvest Biol. Technol. 2013, 77, 111–120. DOI: 10.1016/j.postharvbio.2012.10.010.
- Way, M. L.; Jones, J. E.; Longo, R.; Dambergs, R. G.; Swarts, N. D. A Preliminary Study of Yeast Strain Influence on Chemical and Sensory Characteristics of Apple Cider. Fermentation 2022, 8(9), 455. DOI: 10.3390/fermentation8090455.
- Watts, S.; Migicovsky, Z.; McClure, K. A.; Yu, C. H. J.; Amyotte, B.; Baker, T.; Bowlby, D.; Burgher‐MacLellan, K.; Butler, L.; Donald, R.; et al. Quantifying Apple Diversity: A Phenomic Characterization of Canada’s Apple Biodiversity Collection. Plants. People. Planet. 2021, 3(6), 747–760. DOI: 10.1002/ppp3.10211.
- Dzialo, M. C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K. J. Physiology, Ecology and Industrial Applications of Aroma Formation in Yeast’, FEMS Microbiol. Rev. 2017, 41(1), S95–S128. DOI: https://doi.org/10.1093/femsre/fux031.
- Fărcaș, A. C.; Socaci, S. A.; Chiș, M. S.; Dulf, F. V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27(6), 1987. DOI: 10.3390/molecules27061987.
- Ferrer-Gallego, R.; Hernández-Hierro, J. M.; Rivas-Gonzalo, J. C.; Escribano-Bailón, M. T. Sensory Evaluation of Bitterness and Astringency Sub-Qualities of Wine Phenolic Compounds: Synergistic Effect and Modulation by Aromas. Food Res. Int. 2014, 62, 1100–1107. DOI: 10.1016/j.foodres.2014.05.049.
- Pizarro, C.; Pérez-del-Notario, N.; González-Sáiz, J. M. Headspace Solid-Phase Microextraction for Direct Determination of Volatile Phenols in Cider. J. Sep. Sci. 2009, 32(21), 3746–3754. Available at: DOI: 10.1002/jssc.200900347.
- Yao, H.; Su, H.; Ma, J.; Zheng, J.; He, W.; Wu, C.; Hou, Z.; Zhao, R.; Zhou, Q. Widely Targeted Volatileomics Analysis Reveals the Typical Aroma Formation of Xinyang Black Tea During Fermentation. Food Res. Int. 2023, 164, 112387. DOI: 10.1016/j.foodres.2022.112387.
- Zhang, N.; Jing, T.; Zhao, M.; Jin, J.; Xu, M.; Chen, Y.; Zhang, S.; Wan, X.; Schwab, W.; Song, C.; et al. Untargeted Metabolomics Coupled with Chemometrics Analysis Reveals Potential Non-Volatile Markers During Oolong Tea Shaking. Food Res. Int. 2019, 123, 125–134. DOI: 10.1016/j.foodres.2019.04.053.
- Awale, M.; Liu, C.; Kwasniewski, M. T. Workflow to Investigate Subtle Differences in Wine Volatile Metabolome Induced by Different Root Systems and Irrigation Regimes. Molecules 2021, 26(19), 6010. DOI: 10.3390/molecules26196010.
- Meilgaard, M. C. ‘Aroma Volatiles in Beer: Purification, Flavour, Thresold and Interaction’, Geruch und Geschmackstoffe Internationales Symposium [Preprint]; 1975. https://scholar.google.com/scholar_lookup?title=Aroma+volatiles+in+beer%3A+purification%2C+flavour%2C+thresold+and+interaction&author=Meilgaard%2C+M.C.&publication_year=1975. (accessed Aug 6, 2022).
- Cliff, M.; Stanich, K.; Trujillo, J. M.; Toivonen, P.; Forney, C. F. Determination and Prediction of Odor Thresholds for Odor Active Volatiles in a Neutral Apple Juice Matrix. J. Food Qual. 2011, 34(3), 177–186. DOI: 10.1111/j.1745-4557.2011.00383.x.
- Feng, Y.; Cai, Y.; Fu, X.; Zheng, L.; Xiao, Z.; Zhao, M. Comparison of Aroma-Active Compounds in Broiler Broth and Native Chicken Broth by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV) and Omission Experiment. Food Chem. 2018, 265, 274–280. DOI: 10.1016/j.foodchem.2018.05.043.
- Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods 2020, 9(4), 413. DOI: 10.3390/foods9040413.
- Beckerman, J., et al. Midwest Fruit Pest Management Guide, 2019–2020; 2019. Purdue University: West Lafayette, IN, USA.
- Zhang, J.; Li, L.; Gao, N.; Wang, D.; Gao, Q.; Jiang, S. Feature Extraction and Selection from Volatile Compounds for Analytical Classification of Chinese Red Wines from Different Varieties. Anal. Chim. Acta. 2010, 662(2), 137–142. DOI: 10.1016/j.aca.2009.12.043.
- Harbertson, J. F.; Kennedy, J. A.; Adams, D. O. Tannin in Skins and Seeds of Cabernet Sauvignon, Syrah, and Pinot noir Berries during Ripening. Am. J. Enol. Vitic. 2002, 53(1), 54–59. DOI: 10.5344/ajev.2002.53.1.54.
- Barker, B. T. P. Long Ashton Research Station, 1903–1953. J. Horticultural Sci. 1953, 28(3), 149–151. DOI: 10.1080/00221589.1953.11513779.
- Jun, W.; Kuichuan, S. Variations in Firmness and Sugar Content in “Huanghua” Pear (Pyrus Pyrifolia “Nakai”). J. Horticultural Sci. Biotechnol. 2005, 80(3), 307–312. DOI: 10.1080/14620316.2005.11511935.
- Cardozo, C. J. M.; et al. Physiological and Physico-Chemical Characterization of the Soursop Fruit (Annona muricata L. cv. Elita). Revista Facultad Nacional de Agronomía Medellín 2012, 65(1), 6477–6486.
- Alexander, T. R.; King, J.; Zimmerman, A.; Miles, C. A. Regional Variation in Juice Quality Characteristics of Four Cider Apple (Malus × domestica Borkh.) Cultivars in Northwest and Central Washington. horts. 2016, 51(12), 1498–1502. DOI: 10.21273/HORTSCI11209-16.
- Venkatachalam, K.; Techakanon, C.; Thitithanakul, S. Impact of the ripening stage of wax apples on chemical profiles of juice and cider. ACS omega. 2018, 3(6), 6710–6718.
- Teh, S. L.; Rostandy, B.; Awale, M.; Luby, J. J.; Fennell, A.; Hegeman, A. D. Genetic Analysis of Stilbenoid Profiles in Grapevine Stems Reveals a Major mQTL Hotspot on Chromosome 18 Associated with Disease-Resistance Motifs. Hortic. Res. 2019, 6(1), 121. DOI: 10.1038/s41438-019-0203-x.
- Jie, Y.; Shi, T.; Zhang, Z.; Yan, Q. Identification of Key Volatiles Differentiating Aromatic Rice Cultivars Using an Untargeted Metabolomics Approach. Metabolites 2021, 11(8), 528. DOI: 10.3390/metabo11080528.
- Abrodo, P. A.; Llorente, D. D.; Corujedo, S. J.; de la Fuente, E. D.; Álvarez, M. D. G.; Gomis, D. B. Characterisation of Asturian Cider Apples on the Basis of their Aromatic Profile by High-Speed Gas Chromatography and Solid-Phase Microextraction. Food Chem. 2010, 121(4), 1312–1318. DOI: 10.1016/j.foodchem.2010.01.068.
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the Addition of Different Quantities of Amino Acids to Nitrogen-Deficient Must on the Formation of Esters, Alcohols, and Acids During Wine Alcoholic Fermentation. LWT - Food Sci. Technol. 2008, 41(3), 501–510. DOI: 10.1016/j.lwt.2007.03.018.
- Eleutério dos Santos, C. M.; Pietrowski, G. d A. M.; Braga, C. M.; Rossi, M. J.; Ninow, J.; Machado dos Santos, T. P.; Wosiacki, G.; Jorge, R. M. M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80(6), C1170–C1177. DOI: 10.1111/1750-3841.12879.
- Alberti, A.; Machado dos Santos, T. P.; Ferreira Zielinski, A. A.; Eleutério dos Santos, C. M.; Braga, C. M.; Demiate, I. M.; Nogueira, A. Impact on Chemical Profile in Apple Juice and Cider Made from Unripe, Ripe and Senescent Dessert Varieties. LWT - Food Sci. Technol. 2016, 65, 436–443. DOI: 10.1016/j.lwt.2015.08.045.
- Xu, Y.; Fan, W.; Qian, M. C. Characterization of Aroma Compounds in Apple Cider Using Solvent-Assisted Flavor Evaporation and Headspace Solid-Phase Microextraction. J. Agric. Food Chem. 2007, 55(8), 3051–3057. DOI: 10.1021/jf0631732.
- Rosend, J.; Kuldjärv, R.; Rosenvald, S.; Paalme, T. The Effects of Apple Variety, Ripening Stage, and Yeast Strain on the Volatile Composition of Apple Cider. Heliyon 2019, 5(6), e01953. DOI: 10.1016/j.heliyon.2019.e01953.
- Coelho, E.; Pinto, M.; Bastos, R.; Cruz, M.; Nunes, C.; Rocha, S. M.; Coimbra, M. A. Concentrate Apple Juice Industry: Aroma and Pomace Valuation as Food Ingredients. Appl. Sci. 2021, 11(5), 2443. DOI: 10.3390/app11052443.
- Piškur, J.; Compagno, C. (eds). Molecular Mechanisms in Yeast Carbon Metabolism; Springer: Berlin, Heidelberg, 2014. DOI: 10.1007/978-3-642-55013-3.
- Lambrechts, M. G.; Pretorius, I. S. Yeast and its Importance to Wine Aroma - A Review. SAJEV. 2019, 21(1), 97–129. DOI: 10.21548/21-1-3560.
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical Compositional Characterization of Some Apple Cultivars. Food Chem. 2007, 103(1), 88–93. DOI: 10.1016/j.foodchem.2006.07.030.
- Bicalho, B.; Pereira, A. S.; Aquino Neto, F. R.; Pinto, A. C.; Rezende, C. M. Application of High-Temperature Gas Chromatography − Mass Spectrometry to the Investigation of Glycosidically Bound Components Related to Cashew Apple (Anacardium occidentale L. Var. nanum) Volatiles. J. Agric. Food Chem. 2000, 48(4), 1167–1174. DOI: 10.1021/jf9909252.
- Grigoras, C. G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of Apple Pomace Extracts as a Source of Bioactive Compounds. Ind. Crops Prod. 2013, 49, 794–804. DOI: 10.1016/j.indcrop.2013.06.026.
- López, M. L.; Lavilla, M. T.; Riba, M.; Vendrell, M. Comparison of Volatile Compounds in Two Seasons in Apples: Golden Delicious and Granny Smith. J. Food Qual. 1998, 21(2), 155–166. DOI: 10.1111/j.1745-4557.1998.tb00512.x.
- Mina, M.; Tsaltas, D. Contribution of Yeast in Wine Aroma and Flavour. In Yeast-industrial applications, 2017; pp. 117–134. DOI: 10.5772/intechopen.70656.
- Krstic, M. P.; Johnson, D. L.; Herderich, M. J. Review of Smoke Taint in Wine: Smoke-Derived Volatile Phenols and Their Glycosidic Metabolites in Grapes and Vines as Biomarkers for Smoke Exposure and their Role in the Sensory Perception of Smoke Taint: Review of Smoke Taint in Wine. Aust. J. Grape Wine Res. 2015, 21, 537–553. DOI: 10.1111/ajgw.12183.
- Morales, A. L.; Duque, C. Free and Glycosidically Bound Volatiles in the Mammee Apple (Mammea americana) Fruit. Eur. Food Res. Technol. 2002, 215(3), 221–226. DOI: 10.1007/s00217-002-0546-6.
- USDA Agricultural Marketing Service. Apple Inspection Instructions; 2005. https://www.ams.usda.gov/sites/default/files/media/Apple_Inspection_Instructions%5B1%5D.pdf.