Abstract
This study analyzed hops from 35 fields located in two states (Washington and Oregon) repeatedly over 2 harvest years (2020 and 2021) to determine the impact that hop variety and regional identity, or “terroir,” might have on hops’ dextrin reducing enzymatic potential. Cascade and Mosaic® hops were harvested, kilned, pelletized, and analyzed for dextrin-reducing enzymatic activity using a bench-top dry-hopping assay in a high-dextrin beer. In addition, data for 25 soil, 14 management, 13 climate, and 27 chemistry variables were collected and compared to the enzyme activity results from the bench-top dry-hopping assay. There existed a highly significant difference in enzymatic activity based on hop variety (two sample t-test p-value = 1.18 × 10−14) with Cascade hops being approximately 60% higher on average than Mosaic® hops regardless of growing region or harvest year. The soil and farm management variables also showed statistically significant interactions with enzymatic activity (p-values of 7.82 × 10−9 for Cascade and < 2 × 10−16 for Mosaic®), though there was little clarity with respect to the specific “terroir” variables that might relate to hop creep. Further research is needed to better understand causal interactions between farm, soil, climate, and management practices and dry-hop-induced dextrin-reducing enzymatic activity.
Introduction
Hops (Humulus lupulus) are a primary ingredient in the production of beer contributing desirable aromatic qualities through their essential oils and bitterness and anti-microbial protection through their α- and β-acids.[Citation1–4] Hops balance the residual sweetness of many beers and help create the aromatic signature that defines beer. Hops also contain dextrin-reducing enzymes which can impact beer quality and production efficiency depending on many factors, such as the hop variety, dry-hop contact time, quantity added, the timing of addition and others.[Citation5] Following fermentation, unfermentable longer-chain carbohydrates (usually, oligosaccharides comprising four or more monosaccharides) known as dextrins remain in the beer.[Citation4] When hops are added during or post-fermentation to beer containing residual dextrins, additional fermentable sugars are hydrolyzed from these dextrins that may trigger a slow refermentation. This phenomenon is known colloquially throughout the U.S. brewing industry as “hop creep”[Citation6–8] or “the freshening power of hops.”[Citation4] Hops contain endogenous enzymes that may cleave residual dextrins into shorter-chain carbohydrates, such as glucose, maltose, and maltotriose.[Citation5] Glucose and maltose are easily assimilable sugars for most modern brewer’s yeasts,[Citation9,Citation10] while the consumption of maltotriose is much more strain dependent.[Citation11] When fermentable sugar is consumed by yeast, alcohol and CO2 are produced.[Citation12] Thus, when hops are added to beer containing residual dextrins, the refermentation increases the beer’s alcohol and CO2 content, and in some cases may also result in the production of elevated levels of diacetyl.[Citation13] Consequently, these products may fall out of compliance with alcohol labeling requirements, and the potential for package over-pressurization and failure due to the increased CO2 concentration may result in consumer safety risks.[Citation14] With the advent of modern India pale ales (IPAs) and the craft beer trend towards hoppier beer styles,[Citation15] brewers often use a technique called “dry-hopping”[Citation16,Citation17] to add large volumes of hops to beer at or near the end of fermentation thereby exacerbating the effects of hop creep. Because of these trends and concerns, brewers have widely encouraged research into the factors that might influence the hop creep phenomenon.
There is a growing body of work indicating that the regional identity of hops—the so-called “terroir”—may contribute to differences in hop quality due to the regional growing environment resulting from weather, soil composition, field management practices, and other factors.[Citation18–23] It has been reported that within the same hop variety, the chemical and sensorial properties of hops vary significantly depending on the country of origin. For example, Cascade and Comet hops grown in Yakima, Washington (WA), and Hallertau, Germany displayed differences in analytical and brewing related characteristics.[Citation18] Cascade hops grown in Italy showed analytical differences in bittering capacity and concentration of xanthohumol compared to Cascade hops grown in the US, Germany, and Slovenia.[Citation19] Furthermore, differences in the concentration of the sulfur-based aromatic compound 4-mercapto-4-methyl pentane-2-one (4-MMP)—responsible for a black currant-like aroma in beer—have been identified as a result of the growing region.[Citation20] More recently, Féchir et al. conducted an extensive study analyzing the impact of terroir with respect to hop quality within the Pacific Northwest, looking at Cascade and Mosaic® hops grown in the Yakima Valley, Washington, and the Willamette Valley, Oregon in 2020.[Citation21] The authors observed high variation in both aroma properties and chemical composition between and among Cascade and Mosaic® hops grown in WA and OR. There was also significant field-to-field variation within each region and subregion, which indicates that regional growing conditions and farm management practices play an influential role in hop chemical composition.
Several studies have speculated that regional identity/terroir might influence hop creep potential. Kirkpatrick and Shellhammer demonstrated that dextrin-reducing enzymatic activity differs by hop variety, but they also noted that it may be influenced by growing conditions as indicated by the wide variation between hops grown in different harvest years.[Citation6] Rubottom et al. showed that higher kilning temperatures reduce hop enzymatic activity and reported significant differences in hop enzymatic activity between farms and fields suggesting that there could be potential regional or subregional effects as a result of environment and field management practices.[Citation7] Other studies have shown that farm management decisions regarding maturity and harvest timing may influence dextrin-reducing enzyme activity.[Citation24,Citation25] Each of these studies suggest that growing conditions and farm management decisions could influence regional, subregional, or farm-to-farm variation with respect to hop enzyme activity. A study by Stokholm et al. explored the impact of farm management, soil, and climate on the diastatic potential of Mosaic®, Strata®, and Simcoe®hops grown in Oregon in 2019.[Citation22] However, up to this point, there has not been a comprehensive multi-state, multi-farm, multi-variety, and multi-year study conducted to investigate whether dextrin-reducing enzymatic activity is influenced by regional growing conditions and farm management practices.
The study presented here expands on the previous work on hop regional identity[Citation21] by comparing Cascade and Mosaic® hops grown in 35 fields in two states (Washington and Oregon) over two harvest years (2020 and 2021) to explore the extent to which growing conditions such as location, soil properties and composition, farm management, and climate may impact the dextrin-reducing enzymatic activity of hops with the goal of better understanding the concept of regional identity as a whole and to better control the potential for refermentation in dry-hopped beers.
Materials and methods
Hop collection
Commercially grown hops of the two varieties Cascade and Mosaic® were harvested in 2020 and 2021 from 35 different fields operated according to industrial standards by 22 different hop growers throughout the Pacific Northwest, specifically the Yakima Valley in Washington and the Willamette Valley in Oregon. Samples from 8 Oregon Cascade fields (CO1 − CO8), 8 Oregon Mosaic® fields (MO1 − MO8), 8 Washington Cascade fields (CW1 − CW8), and 11 Washington Mosaic® fields (CW1 − CW11) were collected for each of the 2 harvest years, resulting in 70 hop samples in total.
To ensure that the hop samples from each field were representative of the site based on soil composition, plant vigor, and historical growing abnormalities, specific locations within each field were selected with the assistance of the farm’s owner or manager and a consulting soil scientist. Sampling areas were located well inside the hop fields at a minimum of 20 m from the field’s edge. An area of 5 rows by approximately 20 hills was identified in each field from which a total of 20 plants for Cascade and 15 plants for Mosaic®, respectively, were selected and hand-harvested.
To reduce the confounding influence of harvest maturity, hop samples were harvested no more than 1.5 days prior to the commercial harvest date, which was determined by the grower or farm manager at each location. In 2020, Cascade hops were harvested between August 23 and September 6, and Mosaic® hops were harvested between September 1 and September 19. In 2021, Cascade hops were harvested between August 23 and September 9, and Mosaic® hops were harvested between September 3 and September 20. Also, dry matter measurements, which assess the amount of dry material present in hops at harvest and are often considered an indicator of hop maturity[Citation26] were collected for each sample. The dry matter of the cones at harvest ranged from 19.3% to 26.8% (w/w).
On the same day as harvest, the hops were picked and cleaned of leaves and stems using a mobile small-scale picker (Hopster 5 P, HopsHarvester, Rochester, NY). The cones were then dried shortly thereafter in an industrial scale hop kiln at 57 °C in mesh bags alongside commercial hops of the same variety. Within each of the two states, one specific hop farm was selected for kilning all picked cones within that state in 2020 and 2021 to ensure comparable results between states and years. The cones dried in Oregon were pelletized at Oregon State University while the cones dried in Washington were pelletized on-site at the hop farm performing the kilning using comparable pelleting equipment at each site. The hop pellets exhibited a moisture content between 4.3% and 9.7% (w/w) and were stored vacuum sealed in high-barrier flexible foil pouches at −18 °C prior to analysis. The procedures applied for sampling site selection, harvest, picking, kilning, and pelletizing have previously been described in more detail.[Citation21]
Enzymatic activity analysis
Sample preparation
A lab-scale dry-hopping method was used to measure the enzymatic potential of the hop samples.[Citation7,Citation8] A commercial beer with a high residual dextrin concentration was identified (residual extract 5.5% w/w) and dry-hopped at a rate of 10 g/L. Prior to adding the hops, 0.02 w/v% of sodium azide was added to inhibit interference due to microbiological activity. Samples were incubated statically at 30 °C for 48 h after which the dry-hopped beers were removed from the incubator and sampled. One mL of dry-hopped beer was mixed with one mL of 10% Tris-Base Buffer to halt any further enzymatic activity. The Tris/dry-hopped beer solution was filtered through a 0.45 µm nylon filter (Thermo Scientific, China) into high-performance liquid chromatography (HPLC) vials (VWR International, LLC, Radnor, PA) and frozen to await carbohydrate analysis.
HPLC carbohydrate analysis
Identification and quantitation of the concentrations of fructose, glucose, maltose, and maltotriose were performed on an Agilent 1200 series liquid chromatograph utilizing a Rezex RSO Oligosaccharide Ag + column (200 × 10 mm, particle size 12) with a refractive index detector.[Citation7] 10 µL of the sample were injected with Milli-Q water as the mobile phase at a flow rate of 0.3 mL/minute. The column was held at 80 °C during the run. A five-point calibration was performed using a solution of maltotriose, maltose, glucose, and fructose of known concentration. The response factor was calculated (R2≥0.99) and used to quantitate the concentration of each sugar in the Tris/dry-hopped beer sample.
Hot water extracts
Hops intrinsically contain a small amount of sugar.[Citation6] For accurate reporting of enzymatic potential, the intrinsic sugar content in hops was subtracted from the measured concentration of the prepared samples. A hot water extraction was performed for each hop sample followed by HPLC carbohydrate analysis to determine the intrinsic sugar content of each sample. Hop grist was added to a sodium acetate buffer (0.02 M, pH 4.2) at a rate of 1 g of hops to 100 mL of buffer and incubated at 80 °C for 15 min. The extract was then filtered through a 0.45 µm nylon filter and frozen until carbohydrate analysis was performed via HPLC, as discussed above.
Chemical characteristics, soil, management, and climate correlation analysis
Chemical analyses
The chemical characteristics of the hops were assessed utilizing ASBC-standard methods of analysis to determine total oil concentration, moisture, α- and β-acid concentration by UV-spectroscopy and HPLC, and analysis of selected hop volatile compounds by GC-FID. These selected chemical characteristics are common indicators of hop quality and their relationship with regional identity has been previously reported.[Citation21] Here, their relationship with dextrin-reducing enzymatic activity is analyzed.
Soil samples
For each of the 35 field locations, soil cores were sampled at the same site as the hop sampling locations previously identified at each location. Soil core samples were collected using a bucket auger (76 mm diameter) and sampling to a depth of 1.5 m.
Soil characterization
The soil samples were classified to a series level using USDA standard methods and soil taxonomy and were hand textured by Red Hill Soils, Certified Soil Classifier, Corvallis, Oregon, to estimate the soil texture and the amounts of clay, sand, and silt. Furthermore, the soil parent material was categorized as follows: (1) loamy alluvium, (2) silty alluvium, (3) ice age flood sediments, (4) sandy or gravelly alluvium, and (5) loess over old alluvium/duripan. The available water holding capacity was estimated based on the soil structure and texture as well as the pedotransfer functions, water retention, and laboratory data from the National Cooperative Soil Survey (NCSS).[Citation27]
Soil chemical analysis
Subsurface soil samples were homogenized and analyzed for their amount of organic matter, the cation exchange capacity (CEC), the soil pH as well as the concentrations of Ca, Cu, Fe, H, K, Mg, Mn, Na, P, and Zn and the cation saturation of Ca, H, Mg, and Na by A&L Western Agricultural Laboratories, Portland, OR.
Climate data
Using the PRISM database,[Citation28] a set of climate-related parameters was monitored daily for each of the 35 field locations throughout the time period investigated in this study based on GPS coordinates. This included daily average and cumulative precipitation, daily minimum, maximum, and average temperatures, and daily average vapor pressure deficits, each for the periods of winter (December 1, 2019 − February 29, 2019 and December 1, 2020 − February 28, 2020, respectively), summer (April 1, 2020 − September 30, 2020 and April 1, 2021 − September 30, 2021, respectively) and the growth season, which was specifically determined for each field location based on the respective dates of pruning and harvest. Using the NASA Power database,[Citation29] additional parameters including the average wind speed at 10 m above ground from training to harvest, daily average and cumulative downward thermal infrared radiative fluxes, and daily average insolation clearness indices from pruning to harvest were monitored. Based on these data, several specific climate related system indicators were calculated that are relevant in an agronomic context as previously described.[Citation27]
Field management data
The 35 hop fields investigated in this study were managed for commercial production based on best practices depending on the hop variety, the soil type, and the soil nutrient level. A chemical pruning was performed between March and April and the newly emerged shoots were trained onto 5.5 m strings in May. Drip irrigation was used to apply water and fertilization and the timing and amount of irrigation was managed using on-site weather stations and soil moisture probes. The resulting hop yield, the amount and frequency of applied fungicides and insecticides based on disease and pest pressure as well as the type and quantity of applied fertilization (B, Ca, Cu, Fe, K, Mg, Mn, N, P, S, and Zn) was closely monitored and recorded.[Citation27]
Statistical analysis
Analysis of variance (ANOVA) and Pearson correlation coefficient analyses were performed using XLSTAT® software (version 2018.5.52280, Addinsoft, NY) and R (Version: 3.6.1 GUI 1.70, The R Foundation). R ggplot2 and Microsoft Excel (Version: 16.39, 2020 Microsoft, Redmond, WA) were used for data visualization.
Results and discussion
To characterize the dextrin reducing enzyme activity of hops, the concentrations of glucose and maltose were quantified following a bench top dry-hopping assay in the presence of a strong antimicrobial agent (). Consistent with prior research,[Citation6,Citation7] all Cascade and Mosaic® hop samples displayed measurable enzyme activity demonstrating they are capable of hydrolyzing fermentable sugars from residual dextrins when added to finished beer.
Figure 1. The dextrin reducing enzyme activity of Cascade hops expressed as the concentration of glucose and maltose produced during a dry-hopping assay. Cascade hops were selected from 8 fields in Oregon (CO) and 8 fields in Washington (CW) in harvest years 2020 and 2021.
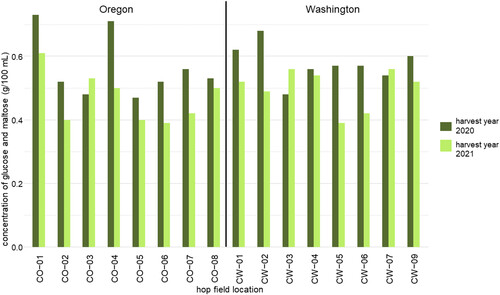
Figure 2. The dextrin reducing enzyme activity of Mosaic® hops expressed as the concentration of glucose and maltose produced during a dry-hopping assay. Mosaic® hops were selected from 8 fields in Oregon (MO) and 11 fields in Washington (MW) in harvest years 2020 and 2021.
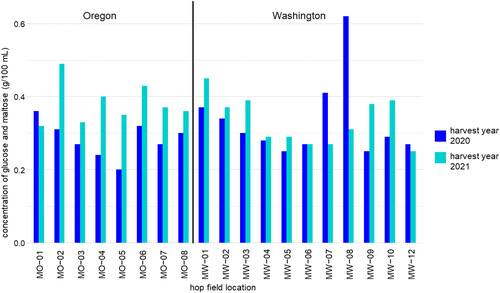
Figure 3. Box and whisker plots showing the hop enzyme activity (expressed as the concentration of glucose and maltose produced during a dry-hopping assay) by variety, state, and harvest year. The average activity for each variety (green representing Cascade, whereas blue represents Mosaic®) is displayed as dotted ⋅⋅⋅⋅⋅ (2020) or dashed—(2021) lines per harvest year. Solid thick lines centered within each box indicate the median activity for each variety × state × harvest year and individual points represent the respective data for each field location.
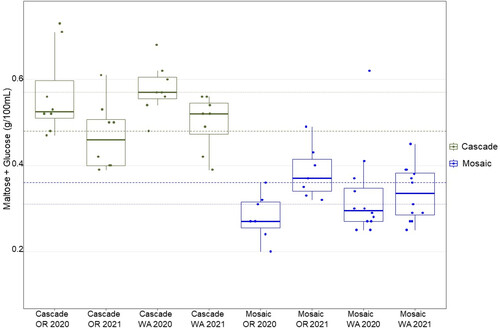
Results from a two sample t-test indicated that the enzyme activity of Cascade hops was significantly higher than Mosaic® hops across all locations and harvest years (p-value = 1.18 × 10 −14, ). Cascade hops produced an average of 0.53 g of maltose and glucose per 100 mL beer in the bench top assay whereas Mosaic® hops produced an average of 0.33 g of maltose and glucose per 100 mL beer. While there have been anecdotal claims from brewers that hop variety may be important in determining the extent to which hop creep occurs in various beer styles and brands, and there have been preliminary findings pointing to a hop variety effect,[Citation6–8] there have been no published studies that thoroughly examine the effect of hop variety under controlled conditions until now. While the current study presented herein is not a comprehensive examination of all major hop varieties, it nonetheless offers strong evidence that hop variety is an important determinant of dextrin reducing enzyme potential by examining two important varieties gathered systematically from two distinct growing areas over 2 harvest years. The relationship between regional identity, harvest year, and enzymatic activity is less clear.
To understand the impact that regional identity might have on the enzymatic potential of Cascade and Mosaic® hops, analysis of variance (ANOVA) was performed by variety examining growing state, harvest year, and farm as independent variables along with their 2-way interactions (). Within each hop variety, there were no statistically significant differences in the hops’ enzymatic activity between states or harvest years. While the graphical representation () suggests that harvest year 2021 may have led to generally lower enzyme activity for Cascade, this observation was not supported by the ANOVA results. Given the lack of significant relationships with harvest year or state of origin, hop variety appears to be one of the best determinants of enzymatic activity. While hop kilning temperature was controlled in the present study, one should keep in mind that on a commercial scale, drying parameters (i.e., temperature) are grower-specific and there is strong evidence that drying temperature plays an important role in the enzyme activity of hops.[Citation7]
Table 1. Analysis of Variance (ANOVA) p-values for state, farm, harvest year, state-by-year interactions, and year-by-farm interactions.
Of the three main effects in this ANOVA, only Farm displayed a significant effect thereby suggesting that soil and farm management practices might be a factor influencing the enzymatic activity of Cascade and Mosaic® hops (). Additionally, there was a significant Year × Farm interaction. That is, the magnitude of the Farm effect on hop enzyme activity depended on harvest year.
To better understand the influence of the Farm and Year × Farm interaction, Pearson’s correlation coefficients were calculated for enzyme activity and each of the 25 soil, 14 management, 13 climate, and 27 chemistry variables, resulting in 79 variables total (; supplemental information). A detailed list of all evaluated variables, along with categorical groupings, is supplied in the supplemental information.
Table 2. Summary of Pearson correlation coefficients showing strong correlations (r > 0.500) between dextrin-reducing enzyme activity and soil, management, climate, and chemistry relationships.
While some relationships showed strong positive or negative correlations, few relationships suggested broader trends with respect to hop variety, state, or harvest year. For instance, the enzymatic activity of Oregon-grown Cascade hops was positively correlated in both harvest years with the amount of organic matter (OM) in the soil and the estimated nitrogen release (ENR), while Washington-grown Cascade enzymatic activity was negatively correlated with OM and ENR regardless of harvest year, albeit weakly in 2021. OM represents the fraction of soil that contains organic material in various states of decomposition, and ENR describes a calculated value estimating the amount of nitrogen that could be released from organic material in the soil over one year. The positive correlation between these measures and enzymatic activity in Oregon-grown Cascade hops indicates that the availability of nitrogen might impact enzymatic activity. However, the results of this study are inconclusive, or possibly dependent on the growing state, as OM and ENR in Washington-grown Cascade hops showed negative correlations with enzymatic activity. The most significant positive correlation (r = 0.961) was observed between enzymatic activity in Oregon-grown 2021 Cascade hops and diurnal flux P-H, a measure indicating the difference between daily maximum and minimum temperatures. A similar strong correlation was found for Mosaic® hops and diurnal flux P-H in 2021 in Oregon, yet correlation magnitude and direction were not consistent across location and harvest years. The strongest negative correlation (r = −0.851) was observed between enzymatic activity in Washington-grown 2021 Mosaic® hops and phosphorus fertilization.
In their 2019 study of Mosaic®, Strata®, and Simcoe® hops grown in Oregon, Stokholm et al. noted that while there was significant variation in the dextrin-reducing enzymatic activity of hops between varieties and fields, there was not a single determinant of enzymatic potential, but rather the potential for a series of unknown interactions between field management, soil, and/or climate.[Citation22] The results from this study suggest a similar interpretation. Several factors that were shown to have a significant relationship with dextrin-reducing enzymatic activity in the Stokholm study (disease pressure—as indicated by quality and quantity of applied fungicides—soil composition, growing degree days, and fertilizer application) did not exhibit the same relationship in this study (). Though there were several singular strong correlations between the farm variables and enzymatic activity in this study, few generalizations can be derived from the data. More research is needed, particularly from multiple years, to better understand the impact that weather, soil, and farm management practices may have with respect to hop-related dextrin-reducing enzymatic activity.
A difficult challenge in terroir studies is accounting for harvest maturity because of differences in growing practices in different regions as well as harvest timing limitations. Harvest maturity has an effect on hop qualities such as oil content and oil composition, and may possibly have an effect on enzyme activity. We do not believe that harvest maturity played a significant role in the present study. The growers in this study harvested their hops when they thought it was appropriate, which may have created some variability in maturity and/or dry matter. It should be emphasized that there is no uniformly accepted metric for determining when hops are ready for harvest. Nonetheless, many growers utilize dry matter measurements as a proxy for estimating or documenting maturity. Dry matter measurements in this study showed mostly insignificant correlations with enzyme activity (). The one instance where there was a weak correlation was for Oregon Cascade in 2021 where dry matter was inversely correlated (−0.551) with enzyme activity.
Conclusion
By repeatedly examining Cascade and Mosaic® hops selected from the same 8–11 sites in each of two prominent growing areas, the Yakima Valley, Washington, and Willamette Valley, Oregon, over two harvest years, this study identified hop variety as being a highly important determinant of hops’ dextrin-reducing enzyme activity. The impact of harvest year and/or growing location was significantly less important and, in many cases, inconsistent relative to the varietal effect. Cascade hops produced an average of 60% more glucose and maltose than Mosaic® hops when added post-fermentation to a high-dextrin beer and thus are more prone to producing the hop creep effect in dry-hopped beers.
The second largest amount of variation in enzyme activity in this study was attributed to the individual farm. This work is consistent with prior work indicating that there is a statistically significant interaction between individual farm, soil, and management variables and enzymatic activity, but a deeper analysis was unable to pinpoint specific factors attributable to an individual farm that broadly affected hop diastatic power. There were several singular strong positive correlations such as between the enzymatic activity in Oregon-grown Cascade hops and the amount of organic material present in the soil or the soil’s estimated nitrogen release. However, the same relationship between organic material present in the soil and estimated nitrogen release and enzymatic activity for Washington-grown Cascade hops showed a negative correlation. The lack of consistent farm management and/or soil effects on hop enzyme activity over multiple harvest years was confirmed by a strong Year × Farm interaction. More research is needed, particularly from multiple harvest years, to better understand causal interactions between soil, climate, and farm management practices and hops’ dextrin-reducing enzymatic activity. The reader must also keep in mind that other factors not included in this study, such as harvest maturity and hop drying temperature, likely play a similarly important role in determining a hop’s ultimate enzyme activity.
Supporting information
A list of variables, their descriptions, and categorical groupings are provided as supplemental information, as well as a correlation matrix showing the relationships between dextrin-reducing enzymatic activity and the explanatory variables.
Supplemental Material
Download MS Word (44.9 KB)Acknowledgments
The authors thank 4-B Farms, B&D Farms, BC Hop Company, Black Star Ranch, B. T. Loftus, C & C Hop Farm, Carpenter Ranches, Coleman Agriculture, F & B Farms, Gasseling Ranches, Greenleaf Hop Farm, John I. Haas, Mark McKay Farms, Oasis Farms, Olsen Brothers Ranches, Perrault Farms, Sauve & Son Farms, Shinn & Son, Sodbuster, Tributary Hop Farms, Van Horn Farms, and Wenas Hop Company for providing hop material. We wish to acknowledge the work of Andy Gallagher, Red Hill Soils, for guiding the selection of each soil and hop sampling site. We also thank Ian Savage, Oregon State University, OR for his skillful assistance in preparing the enzyme assays for analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Literature cited
- Lewis, M. J.; Young, T. W. Brewing; Springer: New York, NY, 2001. DOI: 10.1007/978-1-4615-0729-1.
- Brown, H. T.; Morris, G. Ha. On Certain Functions of Hops Used in the Dry-Hopping of Beers. Brewer’s Guardian 1893, 23, 107–109.
- Janicki, J.; Kotasthane, W. V.; Parker, A.; Walker, T. K. THE DIASTATIC ACTIVITY OF HOPS, TOGETHER WITH A NOTE ON MALTASE IN HOPS. J. Inst. Brew. 1941, 47(1), 24–36. DOI: 10.1002/j.2050-0416.1941.tb06070.x.
- Kirkendall, J. A.; Mitchell, C. A.; Chadwick, L. R. The Freshening Power of Centennial Hops. J. Am. Soc. Brew. Chem. 2018, 76(3), 178–184. DOI: 10.1080/03610470.2018.1469081/SUPPL_FILE/UJBC_A_1469081_SM5495.TXT.
- Cottrell, M. T. A Search for Diastatic Enzymes Endogenous to Humulus lupulus and Produced by Microbes Associated with Pellet Hops Driving “Hop Creep” of Dry Hopped Beer. J. Am. Soc. Brew. Chem. 2022, 81(3), 435–447. DOI: 10.1080/03610470.2022.2084327.
- Kirkpatrick, K. R.; Shellhammer, T. H. A Cultivar-Based Screening of Hops for Dextrin Degrading Enzymatic Potential. J. Am. Soc. Brew. Chem. 2019, 76(4), 247–256. DOI: 10.1080/03610470.2018.1546091.
- Rubottom, L. N.; Lafontaine, S. R.; Hauser, D. G.; Pereira, C.; Shellhammer, T. H. Hop Kilning Temperature Sensitivity of Dextrin-Reducing Enzymes in Hops. J. Am. Soc. Brew. Chem. 2022, 80(1), 75–83. DOI: 10.1080/03610470.2021.1903290.
- Kirkpatrick, K. R.; Shellhammer, T. H. Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus). J. Agric. Food Chem. 2018, 66(34), 9121–9126. DOI: 10.1021/acs.jafc.8b03563.
- Hatanaka, H.; Mitsunaga, H.; Fukusaki, E. Inhibition of Saccharomyces cerevisiae Growth by Simultaneous Uptake of Glucose and Maltose. J. Biosci. Bioeng. 2018, 125(1), 52–58. DOI: 10.1016/J.JBIOSC.2017.07.013.
- D’Amore, T.; Russell, I.; Stewart, G. G. Sugar Utilization by Yeast During Fermentation. J. Ind. Microbiol. 1989, 4(4), 315–323. DOI: 10.1007/BF01577355.
- Zheng, X.; D'Amore, T.; Russell, I.; Stewart, G. G. Factors Influencing Maltotriose Utilization During Brewery Wort Fermentations. J. Am. Soc. Brew. Chem. 1994, 52(2), 41–47. DOI: 10.1094/ASBCJ-52-0041.
- Bruner, J.; Marcus, A.; Fox, G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation 2021, 7(2), 66. DOI: 10.3390/FERMENTATION7020066/S1.
- Bruner, J.; Marcus, A.; Fox, G. Changes in Diacetyl and Amino Acid Concentration during the Fermentation of Dry-Hopped Beer: A Look at Twelve Saccharomyces Species and Strains. J. Am. Soc. Brew. Chem. 2022, 81(2), 242–254. DOI: 10.1080/03610470.2022.2078946.
- Stokholm, A.; Shellhammer, T. H. Hop Creep - Technical Brief. Technical summary prepared for the Brewers Association Educational Publications. 2020. 2 pages. https://www.brewersassociation.org/educational-publications/hop-creep-technical-brief/
- National Beer Sales & Production Data - Brewers Association. https://www.brewersassociation.org/statistics-and-data/national-beer-stats/ (accessed May 23, 2022).
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D.; Dry, H. The Effects of Temperature and Hop Variety on the Bittering Profiles and Properties of Resultant Beers. BrewingScience 2017, 70(11–12), 187–196. DOI: 10.23763/BRSC17-18OLADOKUN.
- Werrie, P. Y.; Deckers, S.; Fauconnier, M. L. Brief Insight into the Underestimated Role of Hop Amylases on Beer Aroma Profiles. J. Am. Soc. Brew. Chem. 2021, 80(1), 66–74. DOI: 10.1080/03610470.2021.1937453.
- Forster, A.; Gahr, A. A Comparison of the Analytical and Brewing Characteristics of Cascade and Comet Hop Varieties as Grown in Yakima (USA) and Hallertau (Germany). BrewingScience 2014, 67(11–12), 137–148. DOI: 10.23763/BrSc14-31forster.
- Rodolfi, M.; Chiancone, B.; Liberatore, C. M.; Fabbri, A.; Cirlini, M.; Ganino, T. Changes in Chemical Profile of Cascade Hop Cones According to the Growing Area. J. Sci. Food Agric. 2019, 99(13), 6011–6019. DOI: 10.1002/JSFA.9876.
- Kishimoto, T.; Kobayashi, M.; Yako, N.; Iida, A.; Wanikawa, A. Comparison of 4-Mercapto-4-Methylpentan-2-One Contents in Hop Cultivars from Different Growing Regions. J. Agric. Food Chem. 2008, 56(3), 1051–1057. DOI: 10.1021/JF072173E.
- Féchir, M.; Weaver, G.; Roy, C.; Shellhammer, T. H. Exploring the Regional Identity of Cascade and Mosaic® Hops Grown at Different Locations in Oregon and Washington. J. Am. Soc. Brew. Chem. 2022, 81(3), 480–492. DOI: 10.1080/03610470.2022.2089010.
- Stokholm, A.; Van Simaeys, K.; Gallagher, A.; Weaver, G.; Shellhammer, T. H. Investigating the Effect of Farm Management, Soil, and Climate on Hop Diastatic Potential. J. Am. Soc. Brew. Chem. 2021, 80(4), 389–400. DOI: 10.1080/03610470.2021.1977902.
- Van Simaeys, K. R.; Féchir, M.; Gallagher, A.; Stokholm, A.; Weaver, G.; Shellhammer, T. H. Examining Chemical and Sensory Differences of New American Aroma Hops Grown in the Willamette Valley, Oregon. J. Am. Soc. Brew. Chem. 2021, 80(4), 370–378. DOI: 10.1080/03610470.2021.1968271.
- Lafontaine, S.; Varnum, S.; Roland, A.; Delpech, S.; Dagan, L.; Vollmer, D.; Kishimoto, T.; Shellhammer, T. Impact of Harvest Maturity on the Aroma Characteristics and Chemistry of Cascade Hops Used for Dry-Hopping. Food Chem. 2019, 278, 228–239. DOI: 10.1016/J.FOODCHEM.2018.10.148.
- Sharp, D. C.; Townsend, M. S.; Qian, Y.; Shellhammer, T. H. Effect of Harvest Maturity on the Chemical Composition of Cascade and Willamette Hops. J. Am. Soc. Brew. Chem. 2018, 72(4), 231–238. DOI: 10.1094/ASBCJ-2014-1002-01.
- Lafontaine, S.; Caffrey, A.; Dailey, J.; Varnum, S.; Hale, A.; Eichler, B.; Dennenlöhr, J.; Schubert, C.; Knoke, L.; Lerno, L.; et al. Evaluation of Variety, Maturity, and Farm on the Concentrations of Monoterpene Diglycosides and Hop Volatile/Nonvolatile Composition in Five Humulus lupulus Cultivars. J. Agric. Food Chem. 2021, 69(15), 4356–4370. DOI: 10.1021/acs.jafc.0c07146.
- Féchir, M.; Gallagher, A.; Weaver, G.; Roy, C.; Shellhammer, T. H. Environmental and Technological Impact Factors on the Regional Identity of Cascade and Mosaic® Hops Grown in the Pacific Northwest. J. Sci. Food Agric. 2023, 103(12), 5802–5810. https://doi.org/10.1002/jsfa.12655
- Prism Climate Group; Northwest Alliance for Computational Science and Engineering. Oregon State University; USDA Risk Management Agency. PRISM Database. https://prism.oregonstate.edu/ (accessed December 22, 2022).
- ProjectTeam, P. The POWER Project. NASA Prediction of Worldwide Energy Resources. https://power.larc.nasa.gov/ (accessed December 22, 2022).

