Abstract
The study aimed to evaluate the hypothesis that chewing is a mechanical and physiological contributor to swallowing, physiologic/pathologic processes of the gastrointestinal tract (GIT), and nutrition-related factors. A search strategy was applied to three different databases to investigate if chewing function in adults affects the swallowing, physiologic/pathologic processes of the GIT, and nutrition-related factors compared to controls with no exposure. The included studies were evaluated for methodological quality and risk of bias and certainty of evidence. The results showed 71 eligible studies. Overall, the results showed that 46 studies supported the hypothesis while 25 refuted it. However, the GRADE analysis showed low to very low certainty of the evidence to support the hypothesis that chewing is an important contributor in the swallowing process, and physiologic/pathologic processes in the GIT. The GRADE analysis also showed a moderate to very low certainty of the evidence to suggest that chewing function contributes to nutrition-related parameters. The overall results of the current study showed that a majority (64.7%) of the studies (46 out of 71) supported the hypothesis. However, robust studies with proper design, adequate sample size, and well-defined outcome parameters are needed to establish conclusive evidence.
Introduction
Mastication or chewing is a rhythmic process with finely synchronized movements coordinated by the components of the masticatory system. The rhythmic act of chewing results primarily in the physical comminution of large aggregates of food morsels and the formation of a soft bolus. The soft bolus, thus formed by mixing food with saliva, is easier and safer to swallow (Lund Citation1991; Mishellany et al. Citation2006; Prinz and Lucas Citation1997). Chewing increases the release of flavor and aromatic molecules that enhance the aroma and taste of food. In addition, the masticatory system is also involved in several other vital functions including respiration (Hasegawa et al. Citation2009), digestion and speech. Besides, there is compelling evidence, mainly from animal studies, indicating that chewing increases the cortical blood flow, particularly to the prefrontal cortex and hippocampus. Chewing may thus play a substantial role in improving subjective alertness, working memory, and cognition; for reviews, please see (Azuma et al. Citation2017; Ono et al. Citation2010; Weijenberg et al. Citation2019; Weijenberg, Scherder, and Lobbezoo Citation2011). Therefore, an impaired masticatory system will result in impaired chewing, which may have adverse effects on multiple bodily functions (Nakata Citation1998; Miquel, Aspiras, and Day Citation2018).
Although chewing and swallowing processes have been extensively studied, their coordination during eating has been overlooked (Yamashita, Sugita, and Matsuo Citation2013). Studies suggest that poor masticatory performance due to removable dentures contributes to impaired swallowing function in older individuals (Son et al. Citation2013; Monaco et al. Citation2012). The absence of dentures in edentulous individuals may alter the anatomical structure and functional movement of the pharynx resulting in poor bolus transport (Yamamoto et al. Citation2013). However, it has been shown that people with higher masticatory performance do not necessarily swallow their food after fewer chewing strokes than those with lower masticatory performance (Gonçalves et al. Citation2021). Subsequently, it has also been reported that people with poor chewing performance do not necessarily swallow food morsels after fewer chewing cycles or poorly prepared blouses (Gonçalves et al. Citation2021; Homsi et al. Citation2021). Therefore, there is a greater need for studies investigating the precise relationship between chewing and swallowing functions.
The role of mastication in the digestive process has not received adequate attention from medical and dental researchers. It is believed that impaired chewing function affects the oral bacterial flora and increases the risk of gastrointestinal pathologies (Tosello et al. Citation2001). Fragments of food that are too large to be digested completely can result in bacterial overgrowth in the colon which may lead to indigestion, bloating and constipation (Mercier and Poitras Citation1992; Ghoshal, Shukla, and Ghoshal Citation2017). Loss of (molar) teeth decreases the trituration ability, resulting in delayed gastric emptying and impaired digestive function (Hattori, Mito, and Watanabe Citation2008). Efficient chewing affects gut signaling and ultimately digestive and absorptive processes (Li et al. Citation2011). Hence, mastication and swallowing not only prepare food to safely pass from the oral cavity to the esophagus but also aid in the subsequent events that occur in the stomach (Kimura et al. Citation2006).
Impaired chewing function may also be an important contributing factor to malnutrition (Marshall et al. Citation2002; Mann, Heuberger, and Wong Citation2013). Edentulous or partially edentulous individuals have been shown to consume significantly lower amounts of fruits and vegetables compared to individuals with a natural dentition (Joshipura, Willett, and Douglass Citation1996). Fiber-rich foods are probably more difficult for them to chew. Due to chewing difficulties older individuals may be particularly vulnerable to nutritional deficiencies because they subsequently exclude high-fiber foods from their diet. A significant association has been demonstrated between impaired chewing ability, food avoidance, and digestive distress in the older adults (Altenhoevel et al. Citation2012). Thorough mastication has been shown to affect postprandial plasma glucose concentrations and increase the absorption of key nutrients (Suzuki et al. Citation2005). As a result of impaired chewing function, chewed food may have varying physical characteristics, which may affect swallowing function, digestion kinetics, and nutritional status (Grundy et al. Citation2015).
Although the process of chewing, swallowing, digestion, and absorption of nutrients processes have all separately been extensively studied, little attention has been given to how they inter-relate. Furthermore, the specific role of chewing on swallowing, digestion, and nutrition related parameters is not clearly evident. Hence, the current systematic review was designed to evaluate the available literature regarding the hypothesis that chewing is a mechanical and physiological “contributor” to the swallowing process, to physiologic/pathologic processes of the gastrointestinal tract (GIT), and to nutrition-related factors, in adult humans.
Material and methods
The current systematic review was performed in accordance with the revised guidelines set forth by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (Page et al. Citation2021). The protocol for the review was registered a priori on the International prospective register of systematic reviews PROSPERO: ID = CRD42018112209.
Search strategy
The current systematic review evaluated original, cross-sectional, clinical cohort, and randomized clinical trials (RCTs) in humans investigating the effects of chewing or physical breakdown of food on swallowing or bolus formation, digestion kinetics, and associated physiologic or pathologic processes of the GIT, and nutritional absorption or other nutrition-related parameters. The Population, Exposure, Comparator, and Outcomes (PECO) was defined as “in adult humans, does chewing function affect the swallowing function, physiologic/pathologic processes of the GIT, and nutrition-related factors compared to controls with no (or other) exposure.” provides a detailed explanation of the inclusion exclusion criteria based on PECOs.
Table 1. PECOs criteria for inclusion and exclusion of studies.
All publications were searched, identified, and selected from three databases, Medline (OVID), Embase (Elsevier), and the Cochrane Library (Wiley). A comprehensive search strategy was planned and applied on 08.09.2018, revised on 07.07.2021 and updated on 16.03.2022 by a team of experienced librarians (see acknowledgments). A detailed description of the search strategy and the MeSH/search terms used for the systematic searches are presented in supplementary file, Supplementary Table 1A–C. There were no restrictions according to the date of publication, but only English-language studies were included. An additional manual search of gray literature with Google Scholar was conducted using free text terms such as chewing and “digestion,” “chewing and gastrointestinal,” and “chewing and nutrition.” Furthermore, references of included studies were also searched for potentially eligible studies through cross-referencing.
Study selection
All the studies identified through the systematic search were imported to the Rayyan web application for systematic reviews (Ouzzani et al. Citation2016). After the removal of the duplicates, two authors assessed the titles and abstracts of the publications for eligibility. The studies were included if the relationship between direct objective assessment of chewing function (Gonçalves et al. Citation2021) or chewing intervention/exposure and one of the measures of swallowing function, physiologic/pathologic processes of the GIT, or nutrition-related factors were presented. The authors categorized all the articles into “included,” “excluded” and “undecided.” All undecided articles were further included or excluded after mutual discussion to consensus. The inter-rater agreement between the two reviewers in excluding or including the studies was assessed using the Intraclass Correlation Coefficient (two-way mixed model with absolute agreement). The results showed good inter-rater agreement (0.793, 95% CI [0.722-0.845], P value ˂ 0.0001). Further, full texts of all the publications published from inception that met or appeared to meet the inclusion criteria were retrieved. All reviews (narrative or systematic), meta-analysis, study protocols, conference abstracts, letters to editors, opinion pieces, commentaries, case reports, invitro, and animal studies were excluded. Further, studies were only included if the chewing function was objectively assessed (i.e., measured chewing performance or chewing efficiency) or if there was an intentional/deliberate change in chewing behavior or similar interventions/exposures. Studies investigating self- or proxy-assessed (indirect) masticatory function (e.g., bite force) (Gonçalves et al. Citation2021) or other subjective assessments measured by questionnaires and interviews evaluating the status of dentition, prosthesis use, and eating behavior, were excluded.
Data extraction and quality assessment
Data extraction was independently performed by three authors (A.K., N.A. and J.J.M.) using customized tables specifically created for the current study. The information about the authors, year, study design, study participants, or participant groups (sample size, age, and sex), intervention/exposure, outcome, major results and conclusions and remarks (if any) were extracted from each study. All selected studies were further evaluated with the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Analytical cross-sectional, cohort, and randomized clinical trials. The purpose of this appraisal is to assess the methodological quality and to determine whether the possibility of bias has been taken into consideration in the design, conduct, and analysis of this study. Accordingly, the included studies were assessed for study quality addressing the risk of bias by two authors (NA and MT). Cumulative scores were calculated by adding the positive responses to the questions in the instrument for each study. Based on the percentage of the cumulative scores of each study, the included studies were rated as high (80%–100%), moderate (60%–79%) or low (less than 60%) quality. The certainty of evidence was assessed with the Grading of Recommendations Assessment Development and Evaluation (GRADE) approach (Guyatt et al. Citation2008).
Results
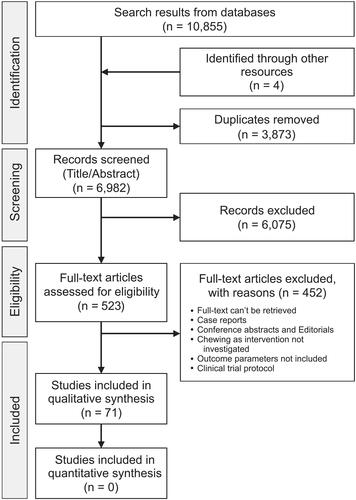
The systematic search strategy using the search terms (Supplementary Table 1) yielded 10,855 publications and 6,982 after removing the duplicates. All the publications were imported to the Rayyan web application for systematic reviews (Ouzzani et al. Citation2016) and the abstracts and titles of all the eligible publications were carefully read. In total, 523 publications were shortlisted and selected for evaluation of their full texts according to the study objectives. Screening of the full text of the publications led to the exclusion of 452 studies because they did not comprehend the study objectives. Further four more articles, identified through cross-referencing, were added to the final list leading to a total of seventy-one articles eligible for the systematic review. The PRISMA flowchart used to guide the selection of the studies is presented in . Out of the seventy-one publications, sixty-two were cross-sectional studies, four cohort studies, and five randomized control trials. Studies were further divided into the effect of chewing on the swallowing process (24 studies), physiologic/pathologic processes in the GIT (10 studies), and nutrition-related factors (37 studies), based on their outcomes. An overview of the data extraction and quality assessment of each study is presented in .
Table 2. Summary of the included studies.
Characteristics of included publications
Although the methods used to measure chewing function, swallowing, digestion, and nutrition-related factors differed in most publications, there were certain similarities that are described below.
Intervention/exposure: chewing assessment methods
Masticatory performance was objectively (direct) assessed by either food comminution, mixing ability, swallowing threshold or other objective chewing tests (Gonçalves et al. Citation2021). Specifically, studies calculated the median particle size distribution of natural and edible test food such as peanuts (Hattori, Mito, and Watanabe Citation2008), almonds (Cassady et al. Citation2009) carrots (Leischker, Kolb, and Felschen-Ludwig Citation2010; Lucas and Luke Citation1986), ham (Pera et al. Citation2002), chicken (Sumonsiri et al. Citation2019) gummy jelly (Iwasaki et al. Citation2022a), representative inedible test food such as Optosil/Optocal tablets (Engelen, Fontijn-Tekamp, and van der Bilt Citation2005; Sierpinska et al. Citation2007; Carretero et al. Citation2011; Flores-Orozco et al. Citation2016; Isabel et al. Citation2015; Sanchez-Ayala, Campanha, and Garcia Citation2013; Campos, Goncalves, and Rodrigues Garcia Citation2014; Goncalves, Campos, and Garcia Citation2015; Amaral et al. Citation2019; Flores-Orozco et al. Citation2020) or chewing gums (Magara et al. Citation2022). Masticatory performance was also objectively assessed by color-changing chewing gums (Furuya et al. Citation2014; Wada, Goto, et al. Citation2017; Koike et al. Citation2013; Okada et al. Citation2010; Kimura et al. Citation2013; Bayram et al. Citation2021; Motokawa et al. Citation2021; Saksono et al. Citation2019), two colors chewing gum mixing test (Müller et al. Citation2013; Liedberg et al. Citation2007; Wallace et al. Citation2018; Liedberg et al. Citation2004; Aquilanti et al. Citation2020; Medeiros et al. Citation2020; de Medeiros et al. Citation2021), or by estimating the glucose in the supernatant liquid collected by washing the chewed pieces of a standardized candy in water (Son et al. Citation2013; Fujimoto et al. Citation2020; Takeshima, Fujita, and Maki Citation2019; Ohta et al. Citation2022; Nishi et al. Citation2022; Sawada et al. Citation2021; Karawekpanyawong et al. Citation2022; Onuki et al. Citation2021).
Chewing as the intervention was investigated by asking edentulous participants to eat with and without dentures (Yamamoto et al. Citation2013) or by experimentally shortening the dental arches (Hattori, Mito, and Watanabe Citation2008). Interventions in normal chewing were performed by eating food (Idris et al. Citation2021) of a different type (Ranawana, Henry, et al. Citation2010), size (Goto et al. Citation2015), consistency (Suzuki et al. Citation2005; Kohyama et al. Citation2007; Saitoh et al. Citation2007; Berretin-Felix et al. Citation2009; Kim and Han Citation2005; Pennings et al. Citation2013; Dubey and Nundy Citation1984) or chewing duration (Lucas and Luke Citation1986; Kochi et al. Citation2021).
Some studies reported the effect of intentional/deliberate changes in the chewing behavior by asking the participants to either chew the test food naturally or with a conscious effort (with volition) (Furuya et al. Citation2014) or to chew and spit out the food after a certain chewing duration (Maeda et al. Citation2020). Similar intentional changes in the chewing behavior were done by asking the participants to eat the test food naturally or deliberately change the number of chewing strokes (Ono et al. Citation2007; Fukatsu et al. Citation2015; Abe and Tsubahara Citation2011; Zhu and Hollis Citation2014a, Citation2014b; Arya et al. Citation2017; Madhu et al. Citation2016) or chewing rate/speed before swallowing (Suzuki et al. Citation2005; Kokkinos et al. Citation2010; Goh, Chatonidi, et al. Citation2021; Goh, Choy, et al. Citation2021; Paphangkorakit et al. Citation2019; Hamada and Hayashi Citation2021).
Outcome measures swallowing assessment methods
The effects of chewing on the swallowing process were investigated in twenty-four original research studies, including twenty-three cross-sectional studies (Yamashita, Sugita, and Matsuo Citation2013; Son et al. Citation2013; Yamamoto et al. Citation2013; Lucas and Luke Citation1986; Engelen, Fontijn-Tekamp, and van der Bilt Citation2005; Magara et al. Citation2022; Furuya et al. Citation2014; Bayram et al. Citation2021; de Medeiros et al. Citation2021; Takeshima, Fujita, and Maki Citation2019; Onuki et al. Citation2021; Goto et al. Citation2015; Kohyama et al. Citation2007; Saitoh et al. Citation2007; Kim and Han Citation2005; Kochi et al. Citation2021; Maeda et al. Citation2020; Fukatsu et al. Citation2015; Mioche, Bourdiol, and Monier Citation2003; Matsuno et al. Citation2017; Abe, Furuya, and Suzuki Citation2011; Fontijn-Tekamp et al. Citation2004; Wada, Kawate, et al. Citation2017) and one cohort study (Berretin-Felix et al. Citation2009). Videofluorography/endoscopy was the most common method to assess swallowing function (Yamashita, Sugita, and Matsuo Citation2013; Son et al. Citation2013; Yamamoto et al. Citation2013; Furuya et al. Citation2014; Saitoh et al. Citation2007; Fukatsu et al. Citation2015; Abe, Furuya, and Suzuki Citation2011; Matsuno et al. Citation2017). Swallowing threshold (Medeiros et al. Citation2020; de Medeiros et al. Citation2021) or oral transit time was assessed by counting the number of chewing strokes until swallowing while eating a standardized portion of test food (Son et al. Citation2013; Lucas and Luke Citation1986; Campos, Goncalves, and Rodrigues Garcia Citation2014; Takeshima, Fujita, and Maki Citation2019; Kim and Han Citation2005; Fontijn-Tekamp et al. Citation2004). Swallowing movement has also been monitored by an array of pressure sensors and sound detected from microphones connected to an amplifier (Kohyama et al. Citation2007). Further the effect of chewing (gum) on swallowing-related neural pathways were also studied by transcranial magnetic stimulation (Magara et al. Citation2022).
Boluses preparation during swallowing was assessed by evaluating the amount of saliva incorporated and mechanical properties of the food bolus (Kochi et al. Citation2021; Fukatsu et al. Citation2015; Goh, Chatonidi, et al. Citation2021; Goh, Choy, et al. Citation2021; Mioche, Bourdiol, and Monier Citation2003; Matsuno et al. Citation2017; Wada, Kawate, et al. Citation2017). Evaluation was performed either by visual inspection of the spat-out food (Berretin-Felix et al. Citation2009) or by fiberoptic endoscopic evaluation/video endoscopy of swallowing movements (Yamamoto et al. Citation2013; Furuya et al. Citation2014; Goto et al. Citation2015; Saitoh et al. Citation2007; Fukatsu et al. Citation2015; Matsuno et al. Citation2017; Abe, Furuya, and Suzuki Citation2011). The criteria for boluses inspection during endoscopy were grinding, mixing, and aggregation of the food bolus (Yamashita, Sugita, and Matsuo Citation2013; Kochi et al. Citation2021; Fukatsu et al. Citation2015; Matsuno et al. Citation2017). Boluses were also evaluated for solidity, adhesiveness, and cohesiveness (Kochi et al. Citation2021; Maeda et al. Citation2020) by the force-time graph obtained from a creep meter (Goto et al. Citation2015). Further, dysphagia risk was evaluated by the Eating Assessment Tool (EAT-10) (Bayram et al. Citation2021) and Dysphagia screening questionnaire (Onuki et al. Citation2021).
Physiologic/pathologic processes of the GIT and assessment methods
The effect of chewing on physiologic/pathologic processes of the GIT was investigated in ten cross-sectional studies (Hattori, Mito, and Watanabe Citation2008; Kimura et al. Citation2006; Pera et al. Citation2002; Sierpinska et al. Citation2007; Koike et al. Citation2013; Dubey and Nundy Citation1984; Arya et al. Citation2017; Pennings et al. Citation2013; Carretero et al. Citation2011; Sumonsiri, Thongudomporn, and Paphangkorakit Citation2018). The effect of chewing on gastric emptying was assessed by a [13C]-labeled acetate breath test (Hattori, Mito, and Watanabe Citation2008; Pera et al. Citation2002; Koike et al. Citation2013), abdominal ultrasonography (Kimura et al. Citation2006) and scintigraphy (Sumonsiri et al. Citation2019). The effect of chewing on gastric acid secretion (Dubey and Nundy Citation1984), and pathologic processes of the GIT was measured by DeMeester score to assess for Gastroesophageal Reflux Disease (GERD) (Arya et al. Citation2017) and the frequency scale for symptoms of gastroesophageal reflux disease (FSSG) (Koike et al. Citation2013). Histopathologic changes of gastric mucosa and severity of Helicobacter pylori infection were scored according to the updated Sydney Classification of Chronic Gastritis (Sierpinska et al. Citation2007). Studies have also evaluated chewing function in clinically diagnosed groups of non-ulcerative functional dyspepsia (Carretero et al. Citation2011).
Nutrition-related parameters
In general, nutrition status is assessed using energy balance indicators, resulting in undernutrition and overweight. Variables such as body weight, body mass index (BMI), body composition, change in blood glucose concentration, and level of certain minerals (e.g., Fe, Mg) in tissue fluids are suggested to be good indicators of nutrition. Accordingly, the effect of chewing on nutrition-related factors was investigated in thirty-seven studies with twenty-nine cross-sectional (Leischker, Kolb, and Felschen-Ludwig Citation2010; Iwasaki et al. Citation2022a; Isabel et al. Citation2015; Sanchez-Ayala, Campanha, and Garcia Citation2013; Campos, Goncalves, and Rodrigues Garcia Citation2014; Flores-Orozco et al. Citation2020; Okada et al. Citation2010; Motokawa et al. Citation2021; Saksono et al. Citation2019; Liedberg et al. Citation2007; Liedberg et al. Citation2004; Aquilanti et al. Citation2020; Medeiros et al. Citation2020; Fujimoto et al. Citation2020; Ohta et al. Citation2022; Nishi et al. Citation2022; Sawada et al. Citation2021; Karawekpanyawong et al. Citation2022; Madhu et al. Citation2016; Kokkinos et al. Citation2010; Goh, Chatonidi, et al. Citation2021; Goh, Choy, et al. Citation2021; Paphangkorakit et al. Citation2019; Hamada and Hayashi Citation2021; Flores-Orozco et al. Citation2016; Kimura et al. Citation2013; Zhu and Hollis Citation2015b; Zhu, Hsu, and Hollis Citation2013), three cohort (Suzuki et al. Citation2005; Goncalves, Campos, and Garcia Citation2015; Amaral et al. Citation2019; Wöstmann et al. Citation2008), and five RCT (Cassady et al. Citation2009; Müller et al. Citation2013; Wallace, McKenna, and Schimmel Citation2017; Zhu, Hsu, and Hollis Citation2014; Ranawana, Monro, et al. Citation2010) design studies. Anthropometric measurements such as BMI were studied in fourteen of the included publications (Leischker, Kolb, and Felschen-Ludwig Citation2010; Flores-Orozco et al. Citation2016; Isabel et al. Citation2015; Sanchez-Ayala, Campanha, and Garcia Citation2013; Flores-Orozco et al. Citation2020; Okada et al. Citation2010; Motokawa et al. Citation2021; Liedberg et al. Citation2007; Fujimoto et al. Citation2020; Karawekpanyawong et al. Citation2022; Idris et al. Citation2021; Zhu and Hollis Citation2014a; Paphangkorakit et al. Citation2019; Zhu and Hollis Citation2015a). Other measures of body composition such as body water percentage, body fat mass, muscle and bone mass (Flores-Orozco et al. Citation2016; Flores-Orozco et al. Citation2020; Medeiros et al. Citation2020; Nishi et al. Citation2022), and body weight and dimensions such as mid-upper-arm circumference, triceps skinfold, and grip strength were also recorded (Okada et al. Citation2010).
Nutritional status was screened by the mini nutritional assessment tool (MNA) to identify the risk of malnutrition (Leischker, Kolb, and Felschen-Ludwig Citation2010; Iwasaki et al. Citation2022a; Saksono et al. Citation2019; Müller et al. Citation2013; Medeiros et al. Citation2020; Ohta et al. Citation2022; Sawada et al. Citation2021; Wöstmann et al. Citation2008; Wallace, McKenna, and Schimmel Citation2017). Food and nutrient intake were assessed with a 24-hour recall survey method (Kwon et al. Citation2017), food frequency questionnaire (Motokawa et al. Citation2021; Saksono et al. Citation2019) or dietary interviews (Amaral et al. Citation2019; Liedberg et al. Citation2007; Aquilanti et al. Citation2020; Karawekpanyawong et al. Citation2022). In one study, food diversity was assessed by an 11-item Food Diversity Score Kyoto (FDSK-11) (Kimura et al. Citation2013). Another study monitored food intake by weighing food plates before and after they were served (Zhu and Hollis Citation2014a).
Blood samples were collected for analysis of nutritional parameters such as blood serum and albumin level (Suzuki et al. Citation2005; Cassady et al. Citation2009; Leischker, Kolb, and Felschen-Ludwig Citation2010; Flores-Orozco et al. Citation2016; Okada et al. Citation2010; Motokawa et al. Citation2021; Müller et al. Citation2013; Wallace et al. Citation2018; Pennings et al. Citation2013; Kokkinos et al. Citation2010; Zhu, Hsu, and Hollis Citation2013; Ranawana, Monro, et al. Citation2010). In particular, the level of blood glucose (Madhu et al. Citation2016), insulin, and Glucagon-like peptide-1 (GLP-1) and diet induced thermogenesis (Hamada and Hayashi Citation2021) was evaluated at regular intervals of time (Suzuki et al. Citation2005; Ranawana, Henry, et al. Citation2010; Madhu et al. Citation2016). Nutrient absorption was also measured in terms of the concentration of gut hormones, insulin, and plasma concentrations of glucose/glucose-dependent insulinotropic peptide (Cassady et al. Citation2009; Goh, Chatonidi, et al. Citation2021; Goh, Choy, et al. Citation2021; Zhu, Hsu, and Hollis Citation2013). Other studies investigated the effect of chewing and swallowing on protein kinetics (Pennings et al. Citation2013), the plasma concentration of gut hormone (Zhu, Hsu, and Hollis Citation2013), and the postprandial response of the orexigenic hormone ghrelin and the anorexigenic peptides (peptide YY and glucagon-like peptide-1) (Kokkinos et al. Citation2010).
Quality assessments, narrative synthesis, and GRADE analysis
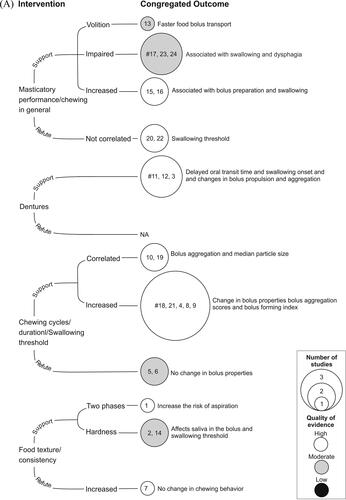
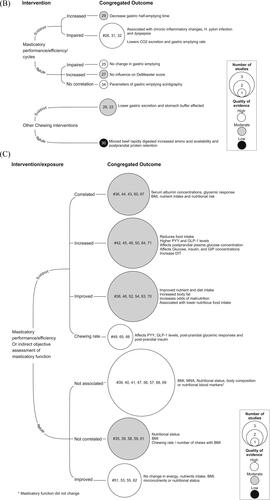
In total, thirty-six studies were rated as high quality (based on JBI criteria) with low risk of bias, twenty-six as moderate, and nine as low-quality studies with high risk of bias (). Studies with a common intervention/exposure and outcome variables were congregated (based on narrative synthesis) and the average score from the quality assessment (JBI criteria) for each congregated outcome was calculated. Thus, the quality assessment of the congregated outcome was based on the average score of the individual studies in the group. The cumulative representation of the intervention/exposure and the congregated outcome variables (swallowing process, physiologic/pathologic processes of the GIT, and nutrition-related parameters) are presented in .
Figure 2. Diagram showing narrative synthesis based on quality assessment using the Joanna Briggs Institute Critical Appraisal Checklist for Analytical cross-sectional, cohort, and randomized clinical trials investigating if chewing is a mechanical or physiological contributor in the (A) process of swallowing, (B) physiologic/pathologic processes in the gastrointestinal tract, and (C) nutrition-related parameters. The interventions/exposure are classified into studies supporting and refuting the hypothesis. The common intervention and outcome parameters are grouped together to form the congregated outcomes. The size of the circles denotes the number of studies, and the color of the circles denote the quality of the congregated outcomes. The numbers in the circles denote the ID number from the studies in . Circles marked with “#” are assessed for certainty of evidence with the Grading of Recommendations Assessment Development and Evaluation (GRADE) approach.
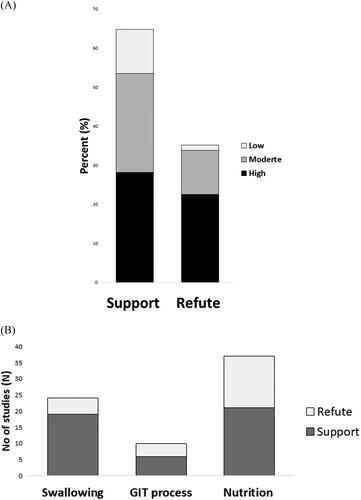
It was hypothesized (as mentioned above) that chewing is an important mechanical and physiological contributor in the swallowing process, physiologic/pathologic processes of the GIT, and in nutrition-related parameters, in humans. Studies were considered to “support the hypothesis” if there was any change (increased or decreased) in the expression of the outcome measures (see, 3.1.2) obtained in the chewing intervention (see 3.1.1). Similarly, a study was adjusted as “refuting the hypothesis” if the chewing intervention did not result in a change in the outcome measures. The overall results of the narrative synthesis showed that 46 out of 71 (64.7%) studies supported the hypothesis. Only 25 (35.3%) studies refuted the hypothesis (). Specifically, the notion that chewing function contributes to either the swallowing process, the process of the GIT, or nutrition-related factors was supported by 19 out of 24; 6 out of 10; and 21 out of 37 investigations, respectively ().
Figure 3. Bar graphs showing the overall A) number of studies (in percent) supporting and refuting the hypothesis that chewing is a mechanical or physiological contributor in the swallowing process, physiologic/pathologic processes of the gastrointestinal tract, and nutrition-related parameters. The different shades of the bars represent the methodological quality of the studies based on the Joanna Briggs Institute (JBI) Critical Appraisal Checklist. B) showing the number of studies (N) supporting and refuting the hypothesis.
The overall certainty of the evidence was evaluated with an adapted GRADE framework. The GRADE analysis was performed for the congregated outcomes consisting of a minimum of 3 studies (Please see circles marked with “#” in .) The evidence for specific outcomes were evaluated based on the study design, risk of bias, inconsistency, indirectness, and imprecision. The overall quality of evidence for all the congregated outcomes () ranged from moderate to very low according to the GRADE criteria (see –C). The level of evidence was downgraded due to limitations in study designs, imprecision (studies with small sample size), and inconsistency because of evident heterogeneity across populations, interventions/exposure, and indirectness.
Table 3. A. Question: Does chewing function affect or contribute to swallowing function
B. Question: Does chewing function affects or contributes to the physiologic/pathologic processes of gastrointestinal tract
C. Question: Does chewing function affects or contributes to nutrition-related factors
Discussion
The current systematic review is a comprehensive analysis of the available literature on chewing as a mechanical and physiological contributor to swallowing and digestive process and nutrition-related factors in adults. The overall results of the current study showed that a majority of the studies (64.7%) supported the hypothesis to some degree. However, the GRADE analysis showed low to very low certainty of the evidence to support the hypothesis that chewing is an important mechanical and physiological contributor in the swallowing process, and in physiologic/pathologic processes in the GIT. Further, the grade analysis also showed a moderate to very low certainty of the evidence to suggest that chewing function contributes to nutrition-related parameters. The results of the study imply that impaired or improved chewing function may affect (impair or improve) swallowing function, digestive process, and nutrition-related parameters. However, more robust studies with proper design, adequate sample size, well defined outcome parameters and large effect size are needed to establish conclusive evidence.
Assessment of chewing function
Measuring chewing function is a challenging task, and various methods have been used to quantify chewing ability (subjective) and chewing efficiency/performance (objective). However, studies have often shown disagreements between subjective and objective measures of chewing function (Pedroni-Pereira et al. Citation2018; Aimaijiang, Otomaru, and Taniguchi Citation2016). For example, in a study by Leischker et al., eighty-one percent of participants reported having no problems with chewing. However, objective tests showed that about 42% of participants were unable to adequately chew a carrot slice (Leischker, Kolb, and Felschen-Ludwig Citation2010). It has been argued that subjective assessment of chewing function in the older individuals provide “overly optimistic” results (Tada and Miura Citation2018). One reason could be that chewing impairment is a gradual process and people often do not recognize the difficulties at early stages. In addition, chewing impairment is often compensated by changing the eating habits and food preparation methods, either by overcooking foods or by avoiding hard-to-chew foods altogether (Pera et al. Citation2002). Therefore, in the current study it was decided to include the studies evaluating chewing efficiency/performance rather than subjectively evaluated chewing ability.
Consequently, in the current study different methods were used to quantify chewing function (see, intervention/exposure in results). Chewing function tests have primarily focused on the ability of the individual to comminute the test food to smaller pieces/particles or kneed two plastic test foods into a bolus. While some studies have used natural foods for comminution tests others have used artificial or representative test foods. Although natural food has the obvious advantage of being normally consumed the texture and size may vary, influencing the quantification of masticatory performance (Isabel et al. Citation2015). Artificial test substances such as silicon based Optosil cubes (comminution test), chewing gums, and molding waxes (mixing ability test) are also accepted to be good alternatives to standardize chewing tasks. However, chewing is a cumulative process of simultaneous food comminution/breakdown and mixing/lubrication of food with saliva (Prinz and Lucas Citation1997). Therefore, it is rational to assume that chewing function can be better quantified by considering the comminution, mixing, and preparation of boluses of test food with a different texture, hardness, or size, in accordance with the previous suggestions (van der Bilt Citation2011; Slagter et al. Citation1992).
Effect of chewing on swallowing
Chewing disintegrates the food morsels and with adequate help of the tongue and orofacial muscles, incorporates saliva (Mioche, Bourdiol, and Monier Citation2003) to form a bolus. Swallowing, like chewing, is one of the most complex sensorimotor behaviors in humans. It was shown that volitional chewing during eating can expedite bolus transport, delay swallowing onset, and enable smooth bolus transit (Furuya et al. Citation2014). Studies have shown a positive correlation between the number of chewing strokes/cycles and the bolus forming index (Yamashita, Sugita, and Matsuo Citation2013; Abe, Furuya, and Suzuki Citation2011). Studies on food bolus analysis have shown higher grinding and mixing scores (Fukatsu et al. Citation2015) and better bolus aggregation in the pharynx (Yamashita, Sugita, and Matsuo Citation2013) in participants who exhibit a higher number of chewing cycles. The relationship between food transport and swallowing initiation is influenced by chewing and the initial consistency and size of the food (Engelen, Fontijn-Tekamp, and van der Bilt Citation2005; Saitoh et al. Citation2007; Fontijn-Tekamp et al. Citation2004; Wada, Kawate, et al. Citation2017). A decrease in food volume per morsel has been suggested to increase the number of chewing strokes, resulting in appropriate bolus properties for swallowing (Goto et al. Citation2015). Impaired mastication is associated with dysphagia (Bayram et al. Citation2021) and causes impaired swallowing in stroke patients (Kim and Han Citation2005). These findings indicate that the greater the number of chewing strokes before swallowing, the better is the bolus formation and the swallowing function.
Older individuals are less capable of preparing boluses in comparison to younger (Matsuno et al. Citation2017). Feeding without dentures delays swallowing onset and also results in a significant delay of pharyngeal swallowing, despite a greater volume of food bolus penetrating the hypopharynx (Yamamoto et al. Citation2013). While removable dentures impair the masticatory performance and increase the oral transit time and oropharyngeal swallow efficiency (Son et al. Citation2013). Replacement of removable partial dentures with fixed implant-supported dentures results in improved chewing and subsequent swallowing function (Berretin-Felix et al. Citation2009). Further, improved chewing function also results in reduced bolus propulsion time and reduced oral residue once the bolus is transported from the oral cavity (Berretin-Felix et al. Citation2009). These studies indicate that chewing performance is indeed increased after oral rehabilitation and this, in turn, results in better swallowing function.
Although chewing is influenced by the size of the morsel and texture of the food these factors may (Saitoh et al. Citation2007; Maeda et al. Citation2020; Mioche, Bourdiol, and Monier Citation2003; Fontijn-Tekamp et al. Citation2004) or may not (Kohyama et al. Citation2007) affect swallowing function. The properties of the bolus such as hardness, cohesiveness, and adhesiveness change as the number of chewing cycles is increased or decreased (Kochi et al. Citation2021; Maeda et al. Citation2020; Fukatsu et al. Citation2015; Matsuno et al. Citation2017). However, studies have also suggested that bolus aggregation scores are independent of the chewing conditions (Saitoh et al. Citation2007; Fukatsu et al. Citation2015). The number of chewing cycles and swallowing threshold were similar in people with implant-supported partial dentures and people with conventional removable partial dentures, although the implant patients showed better masticatory performance in food comminution tests (Campos, Goncalves, and Rodrigues Garcia Citation2014). Masticatory performance did not coincide/correlate with swallowing threshold (de Medeiros et al. Citation2021) in a group of participants of different ages (Takeshima, Fujita, and Maki Citation2019). Video endoscopy during rice-eating showed that older adults required a greater number of chewing cycles but achieved lower bolus aggregation scores than younger controls (Matsuno et al. Citation2017). Bolus aggregation specifically does not seem to depend on the number of chewing cycles or whether the participants chewed food (rice) in a “usual” manner as compared to “chewed-well” (Fukatsu et al. Citation2015). It was also suggested that the urge to swallow food could be triggered by a threshold determined by the food particle size and the degree of lubrication of the food bolus (Prinz and Lucas Citation1995). It is therefore suggested that swallowing is perhaps induced when the bolus properties become suitable for swallowing. Healthy individuals are often capable of adjusting their chewing techniques according to the food texture and food volume (Kohyama et al. Citation2007). In the present study, although majority (N = 19) of the studies supported the hypothesis and indicated that chewing function affects the swallowing process, the certainty of evidence was very low to low (GRADE criteria). Specifically, the grade assessment showed a very low certainty of evidence to support the finding that impaired masticatory performance is associated with swallowing and dysphagia and that chewing with dentures causes delayed oral transit time and changes in bolus propulsion and aggregation. Further there was also low certainty of evidence to suggest that increased chewing cycles or duration or increasing the swallowing threshold causes changes in bolus properties ().
Overall, the included studies on sensorimotor regulation of swallowing behavior and dysphagia have largely focused on the pathophysiology of the pharyngeal phase and little consideration has been given to the oral phase. The oral phase of food processing that involves the breakdown and mixing of food morsels with saliva can be an important determinant of swallowing thresholds and swallowing function (Kim and Han Citation2005). Thus, future studies on the pathophysiology of swallowing should also consider the oral phase.
Effect of chewing on digestion
Gut hormones play an essential physiological and pathophysiological role in regulating energy homeostasis. Ghrelin, an intestinal hormone responsible for regulating appetite, is affected by oral stimulation/mastication (Simonian et al. Citation2005). Therefore, mastication may play an important role in the gut hormone profile and consequently influence energy intake (Li et al. Citation2011). It has been suggested that decreased masticatory efficiency increases the functional load on the stomach and affects the function of the GIT system (Koike et al. Citation2013). The loss of molar teeth decreases the ability to triturate food and results in delayed gastric emptying and impaired digestive function (Hattori, Mito, and Watanabe Citation2008). Accordingly, a higher incidence of digestive complaints is reported in patients with subjective chewing problems and/or discomfort with dentures or ill-fitting dentures (Altenhoevel et al. Citation2012). A significant relationship has been demonstrated between chewing ability and the use of medications for gastrointestinal disorders or digestive complaints (Pera et al. Citation2002). In addition, chewing inefficiency was associated with a higher prevalence of irritable bowel syndrome in adolescents (Khayyatzadeh et al. Citation2018). Hence, in the current study, it was reasonable to hypothesize that chewing is an important contributor to the normal physiological process of the GIT, and impaired chewing would potentially result in digestive distresses and associated problems.
Poor oral trituration has been associated with severe chronic inflammatory changes and H. pylori infection of the gastric mucosa in dyspepsia patients (Sierpinska et al. Citation2007). People with fewer occlusal pairs and decreased masticatory performance presented greater odds of being functional dyspeptics (Carretero et al. Citation2011). The gastric excretion function was significantly lower in a group of older individuals with tubal feeding (without chewing) in comparison to individuals who chewed and swallowed their food (Kimura et al. Citation2006). Further, foods that require chewing have a higher buffering capacity that could neutralize duodenal acidity than foods that do not require chewing (Dubey and Nundy Citation1984). In addition, a randomized controlled, clinical trial reported that minced beef was more rapidly digested and absorbed than beef steak in older adult men (Pennings et al. Citation2013). On one hand, an experimental increase in the number of chewing strokes from 25 to 50 chewing cycles appears to decrease gastric emptying time (Pera et al. Citation2002). On the other hand, reduction in food trituration caused by the shortening of the dental arch did not significantly affect gastrointestinal digestive function (Hattori, Mito, and Watanabe Citation2008). Further, prolonged mealtime (30 min) was associated with a more pronounced anorexigenic gut peptide response than eating very fast (5 min) (Kokkinos et al. Citation2010). People with impaired chewing efficiency show a longer maximum 13CO2 exhalation time as determined by the 13C-acetate breath test (Koike et al. Citation2013) and minced beef is digested more rapidly than beak steak (Pennings et al. Citation2013). Therefore, currently, it is not fully understood if mastication influences gastrointestinal functions. The prevalence of gastrointestinal disorders, especially the ones related to gastrointestinal motor dysfunctions, is high in older people and increases with aging (Dumic et al. Citation2019; Grassi et al. Citation2011). However, although majority (6 out of 10) of the included studies supported the hypothesis, yet the certainty of evidence based on GRADE analysis showed that there was a moderate to very low level of evidence to support and refute the hypothesis on different congregated outcomes (see and ).
Effects of chewing on nutrition-related factors
Nutritional assessments are the prerequisites for interpretation of data that determines the nutritional status of an individual (or a group). In the current study, different methods were used for assessments of nutrition-related parameters. Compromised oral health and impaired chewing function are suggested to be important determinants of nutritional status. These determinants may influence the eating habits and the supply of key ingredients, especially in the older adults (Motokawa et al. Citation2021; Sheiham and Steele Citation2001). Previous studies have shown that chewing efficiency/performance influences food choices (Mioche, Bourdiol, and Peyron Citation2004) and, subsequently, dietary and nutrient intake, especially in older adults (Goncalves, Campos, and Garcia Citation2015; Motokawa et al. Citation2021). People with chewing difficulties tend to consume a lower amount of fruits and vegetables resulting in lower nutrients such as calcium (Kwon et al. Citation2017), potassium, and vitamin C, compared to controls (Kimura et al. Citation2013; Kwon et al. Citation2017). Further, community-dwelling older individuals with lower chewing efficiency tend to consume lesser food variety and lesser frequency of beans, vegetables, seaweeds, and nuts (Kimura et al. Citation2013). One study showed that the replacement of conventional removable partial dentures with fixed implant-supported dentures resulted in better masticatory performance and an increased protein, fiber, and carbohydrate intake (Campos, Goncalves, and Rodrigues Garcia Citation2014). Recently, a randomized control trial reported that nutritional status could be predicted from masticatory performance (Wallace et al. Citation2018). Further, it was suggested that oral hypofunction (which includes masticatory performance in food commination tests) contributed to greater odds of presence and severity of malnutrition (Iwasaki et al. Citation2022b). Based on these findings, it was hypothesized that chewing function may also affect the nutrition status in adults.
BMI is one of the low-cost, easy to measure, and widely used indicators of nutritional status, nutritional risk, and obesity (Nuttall Citation2015). High correlations have been established between BMI, body fat, and morbidity and mortality (Flegal et al. Citation2013; Kong et al. Citation2017). Negative correlations between BMI and the number of chewing cycles, and chewing duration have been reported (Zhu and Hollis Citation2015b). Studies have also shown significant associations between chewing function, body fat (Sanchez-Ayala, Campanha, and Garcia Citation2013), and anthropometric measurements (Okada et al. Citation2010). Masticatory efficiency was suggested to be a risk factor for increased body fat (Sanchez-Ayala, Campanha, and Garcia Citation2013). Underweight people were shown to chew slowly and more asymmetrically than normal weight or obese counterparts (Flores-Orozco et al. Citation2016). Chewing efficiency and swallowing threshold were significantly associated with BMI and people with higher BMI had higher scores of chewing performance (Isabel et al. Citation2015; Sanchez-Ayala, Campanha, and Garcia Citation2013). The reported correlations and associations potentially indicate that chewing function can influence BMI in accordance with the previous studies (Motokawa et al. Citation2021; Okubo et al. Citation2018; White et al. Citation2015).
Chewing function was reported to be a good predictor of serum albumin concentrations; an important biomarker of nutrition (Okada et al. Citation2010). The degree of mastication and chewing rate was shown to be significantly correlated with the glycemic response (Ranawana, Henry, et al. Citation2010; Goh, Chatonidi, et al. Citation2021), PP insulin and satiety responses (Goh, Chatonidi, et al. Citation2021). Thorough mastication affects the postprandial glucose concentration and absorption of nutrients (Suzuki et al. Citation2005; Madhu et al. Citation2016). The initial postingestive glucagon-like peptide-1 concentrations were also reported to be increased after chewing almonds for 40 strokes as compared to 25 strokes (Cassady et al. Citation2009). Similarly, it was reported that minced meat results in increased amino acid availability and greater postprandial protein retention but does not result in greater postprandial muscle protein synthesis rates (Pennings et al. Citation2013). Therefore, food texture including hardness is an important factor that influences not only the chewing behavior (Grigoriadis, Johansson, and Trulsson Citation2014; Grigoriadis et al. Citation2019; Grigoriadis and Trulsson Citation2018) but also the bolus formation (Engelen, Fontijn-Tekamp, and van der Bilt Citation2005; Saitoh et al. Citation2007; Mioche, Bourdiol, and Monier Citation2003), digestion (Pennings et al. Citation2013), and nutrition.
Contradictory to the above-mentioned findings it was shown that inadequate dietary intake was independent of dental status and both, masticatory ability, and performance (Liedberg et al. Citation2007). Studies have reported that neither chewing rate nor the number of chews per morsel were correlated with BMI (Paphangkorakit et al. Citation2019). Studies have also reported that masticatory performance (or improved masticatory performance) is not associated with nutritional status (Flores-Orozco et al. Citation2016; Amaral et al. Citation2019; Aquilanti et al. Citation2020), BMI (Flores-Orozco et al. Citation2020; Liedberg et al. Citation2007; Fujimoto et al. Citation2020) or body composition (Medeiros et al. Citation2020). There was no significant difference in energy and nutrients intake or BMI despite improved masticatory performance due to fixed dentures as compared to removable (Saksono et al. Citation2019; Liedberg et al. Citation2004). The postprandial plasma glucose concentration was reduced with thorough mastication (Suzuki et al. Citation2005) although it could be anticipated that thorough mastication increases the plasma glucose concentration. Further, even though the hospitalized geriatric patients with acute diseases and with good chewing function had higher scores on the mini nutritional assessment (MNA) and had higher concentrations of vitamins and trace elements, yet there were no significant correlations. (Leischker, Kolb, and Felschen-Ludwig Citation2010). The authors also reported that patients with poor masticatory function tended to show lower (yet not significant) serum levels of Vitamin B1, Niacin, Vitamin C, Vitamin A, and Selenium (Leischker, Kolb, and Felschen-Ludwig Citation2010). Further, it is also shown that dental status and self-assessed masticatory ability have little influence on the dietary selection in older individuals (Österberg et al. Citation2002). Hence, it is suggested that masticatory efficiency is not the only factor affecting or determining the nutritional status and restoring the dental status alone apparently cannot improve nutritional status (Müller et al. Citation2013; Wöstmann et al. Citation2008). Chewing problems may be one of several factors that make older adults susceptible to nutrition deficiencies. Based on the narrative synthesis, 21 studies (out of 37) supported the hypothesis, whereas 16 of them refuted it (). However, the certainty of evidence based on GRADE analysis on the congregated outcomes () showed that there was a moderate level of evidence to support of the chewing rate affects gut hormones ().
Strengths, limitations, and implications
A meta-analysis was not appropriate to implement in the present study as selected studies are largely heterogeneous in terms of study participants involved, interventions/exposures, and outcomes to provide a reasonable overview (see, intervention/exposure 3.1.1 and outcome 3.1.2 above). Accordingly, findings from the included studies are interpreted using narrative synthesis, an approach that uses words and text to summarize and explain results from multiple studies (Yellowitz Citation2016). In the current study since there were different chewing interventions and several outcomes it was rather pragmatic to adopt this approach. A majority of the studies in the current systematic review use a cross-sectional design (N = 62) and can therefore not demonstrate causal relationships between chewing impairment and the assessed outcome variables. The quality assessment of the individual studies was evaluated to determine the bias in the design, conduct, and analysis of the data. Accordingly, it was found that 7 studies were of low quality. In spite of this, all studies that met the inclusion criteria, regardless of quality or small sample size, were included in the analysis to provide a holistic overview of the topic. The figures () are formed by the narrative synthesis of the congregated outcomes and provide a logical representation of the cluster of studies supporting and refuting the hypothesis. Further, the GRADE criteria were only applied to the congregated outcomes (see “#” in ) with three or more studies for evaluating the certainty of evidence. This approach may provide a more focused impression of the cluster of evidence and is considered as a positive attribute of the current study.
Despite a comprehensive hypothesis, only a limited number of studies (N = 71) were found eligible according to the inclusion criteria. Therefore, there is an undeniable need to establish strong evidence through large studies with adequate sample size and power. Given that chewing is an important step in food oral processing, multidisciplinary collaboration is needed to construct high-quality studies with broad perspectives on the issue. A major problem in clinical practice is that chewing and swallowing dysfunctions are managed by several clinical specialties and the interdisciplinary collaboration is often limited (Yellowitz Citation2016). A striking observation is that although chewing and swallowing functions are very closely related, dentists and speech therapists rarely work together. Similarly, there is also lack of strong collaboration between dentists and dieticians. Furthermore, modern dentistry is primarily directed toward structural rehabilitation of the dentition, and not toward functional rehabilitation of the masticatory system. The problem is further accentuated because, at present, there are no reliable, clinical, and easy to use tests to identify the signs of impaired chewing and swallowing behavior. Thus, this area of research could be better served by interdisciplinary collaborations. Moreover, a switch of tracks toward functional rehabilitation instead of structural restoration is warranted.
Conclusion
The results of the current systematic review study showed that the hypothesis was overall supported by forty-six studies and refuted by twenty-five. The results of the study provide pragmatic and preliminary indication on the role of chewing in the measured outcome parameters. However, the overall results also suggest a “moderate” to “very low” certainty of the evidence to support the hypothesis that chewing is an important mechanical and physiological contributor in the swallowing process, and physiologic/pathologic processes in the GIT and various nutrition-related parameters, in humans. It is suggested that chewing function, gastrointestinal disorders, and malnutrition have a complex multifactorial etiology with at least partly similar risk factors (Altenhoevel et al. Citation2012). Impaired chewing function may result from functional disturbances, tooth loss, or poor dentures; however, the extent to which these factors may impact an individual’s overall health is also dependent on somatic, social, and psychological factors. This review also highlights the lack of interdisciplinary collaborations between different specialties of healthcare in constructing well designed studies. Chewing function is one of the important physiological contributors to overall general health and general quality of life. Well-designed studies able to show causality, with sufficient sample size, studying the relationship chewing function as exposer and swallowing, GIT system, and nutrition-related factors as outcomes are needed to establish stronger, conclusive evidence for their complex inter-play.
Acknowledgements
We would like to thank Emma-Lotta Säätelä, Susanne Gustafsson & Sabina Gillsund, from the Karolinska Institutet library for their effortless support in constructing the search strategies and implementing the literature search.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data can be obtained from the corresponding author on reasonable request.
Additional information
Funding
References
- Abe, H., and A. Tsubahara. 2011. Observation of arytenoid movement during laryngeal elevation using videoendoscopic evaluation of swallowing. Dysphagia 26 (2):150–4. doi: 10.1007/s00455-010-9285-1.
- Abe, R., J. Furuya, and T. Suzuki. 2011. Videoendoscopic measurement of food bolus formation for quantitative evaluation of masticatory function. Journal of Prosthodontic Research 55 (3):171–8. doi: 10.1016/j.jpor.2010.12.002.
- Aimaijiang, Y., T. Otomaru, and H. Taniguchi. 2016. Relationships between perceived chewing ability, objective masticatory function and oral health-related quality of life in mandibulectomy or glossectomy patients with a dento-maxillary prosthesis. Journal of Prosthodontic Research 60 (2):92–7. doi: 10.1016/j.jpor.2015.07.005.
- Altenhoevel, A., K. Norman, C. Smoliner, and I. Peroz. 2012. The impact of self-perceived masticatory function on nutrition and gastrointestinal complaints in the elderly. The Journal of Nutrition, Health & Aging 16 (2):175–8. doi: 10.1007/s12603-011-0342-8.
- Amaral, C. F. D., G. A. Souza, M. A. Pinheiro, C. H. Campos, and R. Garcia. 2019. Sensorial ability, mastication and nutrition of single-implant overdentures wearers. Brazilian Dental Journal 30 (1):66–72. doi: 10.1590/0103-6440201902086.
- Aquilanti, L., S. Alia, S. Pugnaloni, E. Coccia, M. Mascitti, A. Santarelli, L. Limongelli, G. Favia, M. Mancini, A. Vignini, et al. 2020. Impact of elderly masticatory performance on nutritional status: An observational study. Medicina 56 (3):130. doi: 10.3390/medicina56030130.
- Arya, V., S. Agarwal, S. Singh, C. Sison, and K. A. Gupta. 2017. The effect of increased chewing strokes on the DeMeester score. Diseases of the Esophagus 30 (5):1–5. doi: 10.1093/dote/dow016.
- Azuma, K., Q. Zhou, M. Niwa, and K.-Y. Kubo. 2017. Association between mastication, the hippocampus, and the HPA axis: A comprehensive review. International Journal of Molecular Sciences 18 (8):1687. doi: 10.3390/ijms18081687.
- Bayram, H. M., F. Ilgaz, S. Serel-Arslan, N. Demir, and N. Rakıcıoğlu. 2021. The relationship between dysphagia, oral health, masticatory performance and activities of daily living in elderly individuals as assessed by the Eating Assessment Tool. Progress in Nutrition 23 (1):e2021005.
- Berretin-Felix, G., W. M. Machado, K. F. Genaro, and N. Filho. 2009. H. Effects of mandibular fixed implant-supported prostheses on masticatory and swallowing functions in completely edentulous elderly individuals. The International Journal of oral & maxillofacial implants 24 (1):110–7.
- Campos, C. H., T. M. S. V. Goncalves, and R. C. M. Rodrigues Garcia. 2014. Implant retainers for free-end removable partial dentures affect mastication and nutrient intake. Clinical Oral Implants Research 25 (8):957–61. doi: 10.1111/clr.12165.
- Carretero, D., A. Sanchez-Ayala, A. Rodriguez, M. O. Lagravere, T. M. S. V. Goncalves, and R. C. M. R. Garcia. 2011. Relationship between non-ulcerative functional dyspepsia, occlusal pairs and masticatory performance in partially edentulous elderly persons. Gerodontology 28 (4):296–301. doi: 10.1111/j.1741-2358.2010.00377.x.
- Cassady, B. A., J. H. Hollis, A. D. Fulford, R. V. Considine, and R. D. Mattes. 2009. Mastication of almonds: Effects of lipid bioaccessibility, appetite, and hormone response. The American Journal of Clinical Nutrition 89 (3):794–800. doi: 10.3945/ajcn.2008.26669.
- de Medeiros, M. M. D., O. M. C. de Figueredo, M. A. Pinheiro, L. F. S. de Oliveira, R. L. Wanderley, E. C. F. de Araújo, Y. W. Cavalcanti, and R. C. M. Rodrigues Garcia. 2021. Prosthetic rehabilitation status, dental prosthesis functionality and masticatory function in nursing home residents. Gerodontology doi: 10.1111/ger.12587.
- Dubey, P., and S. Nundy. 1984. Mastication and acid secretion. Postgraduate Medical Journal 60 (702):272–4. doi: 10.1136/pgmj.60.702.272.
- Dumic, I., T. Nordin, M. Jecmenica, M. Stojkovic Lalosevic, T. Milosavljevic, and T. Milovanovic. 2019. Gastrointestinal tract disorders in older age. Canadian Journal of Gastroenterology & Hepatology 2019:6757524. doi: 10.1155/2019/6757524.
- Engelen, L., A. Fontijn-Tekamp, and A. van der Bilt. 2005. The influence of product and oral characteristics on swallowing. Archives of oral biology 50 (8):739–46. doi: 10.1016/j.archoralbio.2005.01.004.
- Flegal, K. M., B. K. Kit, H. Orpana, and B. I. Graubard. 2013. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 309 (1):71–82. doi: 10.1001/jama.2012.113905.
- Flores-Orozco, E. I., G. E. Tiznado-Orozco, O. D. Osuna-Gonzalez, C. L. Amaro-Navarrete, B. Rovira-Lastra, and J. Martinez-Gomis. 2016. Lack of relationship between masticatory performance and nutritional status in adults with natural dentition. Archives of oral biology 71:117–21. doi: 10.1016/j.archoralbio.2016.07.008.
- Flores-Orozco, E. I., P. M. Pérez-Rodríguez, E. A. Flores-Mendoza, J. M. Flores-Ramos, B. Rovira-Lastra, and J. Martinez-Gomis. 2020. Nutritional status and masticatory function of the indigenous compared with non-indigenous people of Nayarit, Mexico. Archives of oral biology 115:104731. doi: 10.1016/j.archoralbio.2020.104731.
- Fontijn-Tekamp, F. A., A. P. Slagter, A. Van der Bilt, M. A. Van’t Hof, W. Kalk, and J. A. Jansen. 2004. Swallowing thresholds of mandibular implant-retained overdentures with variable portion sizes. Clinical Oral Implants Research 15 (3):375–80. doi: 10.1111/j.1600-0501.2004.01006.x.
- Fujimoto, K., H. Suito, K. Nagao, and T. Ichikawa. 2020. Does masticatory ability contribute to nutritional status in older individuals? International Journal of Environmental Research and Public Health 17 (20):7373.
- Fukatsu, H., K. Nohara, Y. Kotani, N. Tanaka, K. Matsuno, and T. Sakai. 2015. Endoscopic evaluation of food bolus formation and its relationship with the number of chewing cycles. Journal of Oral Rehabilitation 42 (8):580–7. doi: 10.1111/joor.12290.
- Furuya, J., A. Hara, T. Nomura, and H. Kondo. 2014. Volitional chewing with a conscious effort alters and facilitates swallowing during feeding sequence. Journal of Oral Rehabilitation 41 (3):191–8. doi: 10.1111/joor.12140.
- Ghoshal, U. C., R. Shukla, and U. Ghoshal. 2017. Small intestinal bacterial overgrowth and irritable bowel syndrome: A bridge between functional organic dichotomy. Gut and Liver 11 (2):196–208. doi: 10.5009/gnl16126.
- Goh, A. T., G. Chatonidi, M. Choy, S. Ponnalagu, M. Stieger, and C. G. Forde. 2021. Impact of individual differences in eating rate on oral processing, bolus properties and post-meal glucose responses. Physiology & Behavior 238:113495. doi: 10.1016/j.physbeh.2021.113495.
- Goh, A. T., J. Y. M. Choy, X. H. Chua, S. Ponnalagu, C. M. Khoo, C. Whitton, R. M. van Dam, and C. G. Forde. 2021. Increased oral processing and a slower eating rate increase glycaemic, insulin and satiety responses to a mixed meal tolerance test. European Journal of nutrition 60 (5):2719–33. doi: 10.1007/s00394-020-02466-z.
- Gonçalves, T. M. S. V., M. Schimmel, A. van der Bilt, J. Chen, H. W. van der Glas, K. Kohyama, M. Hennequin, M.-A. Peyron, A. Woda, C. R. Leles, et al. 2021. Consensus on the terminologies and methodologies for masticatory assessment. Journal of oral rehabilitation 48 (6):745–61. doi: 10.1111/joor.13161.
- Goncalves, T., C. H. Campos, and R. Garcia. 2015. Effects of implant-based prostheses on mastication, nutritional intake, and oral health-related quality of life in partially edentulous patients: A paired clinical trial. The International Journal of oral & maxillofacial implants 30 (2):391–6. doi: 10.11607/jomi.3770.
- Goto, T., A. Nakamich, M. Watanabe, K. Nagao, M. Matsuyama, and T. Ichikawa. 2015. Influence of food volume per mouthful on chewing and bolus properties. Physiology & Behavior 141:58–62. doi: 10.1016/j.physbeh.2015.01.007.
- Grassi, M., L. Petraccia, G. Mennuni, M. Fontana, A. Scarno, S. Sabetta, and A. Fraioli. 2011. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutricion Hospitalaria 26 (4):659–68.
- Grigoriadis, A., A. Kumar, M. K. Åberg, and M. Trulsson. 2019. Effect of Sudden Deprivation of Sensory Inputs From Periodontium on Mastication. Frontiers in neuroscience 13:1316.
- Grigoriadis, A., and M. Trulsson. 2018. Excitatory drive of masseter muscle during mastication with dental implants. Scientific Reports 8 (1):8597. doi: 10.1038/s41598-018-26926-z.
- Grigoriadis, A., R. S. Johansson, and M. Trulsson. 2014. Temporal profile and amplitude of human masseter muscle activity is adapted to food properties during individual chewing cycles. Journal of oral rehabilitation 41 (5):367–73. doi: 10.1111/joor.12155.
- Grundy, M. M., T. Grassby, G. Mandalari, K. W. Waldron, P. J. Butterworth, S. E. Berry, and P. R. Ellis. 2015. Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. The American Journal of Clinical Nutrition 101 (1):25–33. doi: 10.3945/ajcn.114.088328.
- Guyatt, G. H., A. D. Oxman, G. E. Vist, R. Kunz, Y. Falck-Ytter, P. Alonso-Coello, and H. J. Schünemann. 2008. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research ed.) 336 (7650):924–6. doi: 10.1136/bmj.39489.470347.AD.
- Hamada, Y., and N. Hayashi. 2021. Chewing increases postprandial diet-induced thermogenesis. Scientific Reports 11 (1):23714. doi: 10.1038/s41598-021-03109-x.
- Hasegawa, Y., J. Sakagami, T. Ono, K. Hori, M. Zhang, and Y. Maeda. 2009. Circulatory response and autonomic nervous activity during gum chewing. European Journal of oral sciences 117 (4):470–3. doi: 10.1111/j.1600-0722.2009.00637.x.
- Hattori, Y., Y. Mito, and M. Watanabe. 2008. Gastric emptying rate in subjects with experimentally shortened dental arches: A pilot study. Journal of Oral Rehabilitation 35 (6):402–7. doi: 10.1111/j.1365-2842.2007.01789.x.
- Homsi, G., A. Kumar, N. Almotairy, E. Wester, M. Trulsson, and A. Grigoriadis. 2021. Assessment of masticatory function in older individuals with bimaxillary implant-supported fixed prostheses or with a natural dentition: A case-control study. The Journal of Prosthetic Dentistry. doi: 10.1016/j.prosdent.2021.08.023.
- Idris, G., C. Smith, B. Galland, R. Taylor, C. J. Robertson, H. Bennani, and M. Farella. 2021. Relationship between chewing features and body mass index in young adolescents. Pediatric Obesity 16 (5):e12743.
- Isabel, C. A. C., M. R. Moysés, A. van der Bilt, G. H. Gameiro, J. C. R. Ribeiro, and L. J. Pereira. 2015. The relationship between masticatory and swallowing behaviors and body weight. Physiology & Behavior 151:314–9. doi: 10.1016/j.physbeh.2015.08.006.
- Iwasaki, M., K. Motokawa, Y. Watanabe, M. Shirobe, Y. Ohara, A. Edahiro, H. Kawai, Y. Fujiwara, H. Kim, K. Ihara, et al. 2022a. Oral hypofunction and malnutrition among community—dwelling older adults: Evidence from the Otassha study. Gerodontology 39 (1):17–25. doi: 10.1111/ger.12580.
- Iwasaki, M., K. Motokawa, Y. Watanabe, M. Shirobe, Y. Ohara, A. Edahiro, et al. 2022b. Oral hypofunction and malnutrition among community—Dwelling older adults: Evidence from the Otassha study. Gerodontology.
- Joshipura, K. J., W. C. Willett, and C. W. Douglass. 1996. The impact of edentulousness on food and nutrient intake. Journal of the American Dental Association (1939) 127 (4):459–67. doi: 10.14219/jada.archive.1996.0237.
- Karawekpanyawong, R., K. Nohno, Y. Kubota, and H. Ogawa. 2022. Oral health and nutritional intake in community-dwelling 90-year-old japanese people: A cross-sectional study. Gerodontology.
- Khayyatzadeh, S. S., S. M. R. Kazemi-Bajestani, S. J. Mirmousavi, M. Heshmati, S. Khoshmohabbat, G. A. Ferns, and M. Ghayour-Mobarhan. 2018. Dietary behaviors in relation to prevalence of irritable bowel syndrome in adolescent girls. Journal of gastroenterology and hepatology 33 (2):404–10. doi: 10.1111/jgh.13908.
- Kim, I. S., and T. R. Han. 2005. Influence of mastication and salivation on swallowing in stroke patients. Archives of Physical Medicine and Rehabilitation 86 (10):1986–90. doi: 10.1016/j.apmr.2005.05.004.
- Kimura, Y., H. Ogawa, A. Yoshihara, T. Yamaga, T. Takiguchi, T. Wada, R. Sakamoto, Y. Ishimoto, E. Fukutomi, W. Chen, et al. 2013. Evaluation of chewing ability and its relationship with activities of daily living, depression, cognitive status and food intake in the community-dwelling elderly. Geriatrics & Gerontology International 13 (3):718–25. doi: 10.1111/ggi.12006.
- Kimura, Y., M. Nomura, Y. Sawada, N. Muraoka, N. Kohno, and S. Ito. 2006. Evaluation of the effects of mastication and swallowing on gastric motility using electrogastrography. The Journal of Medical Investigation 53 (3-4):229–37. doi: 10.2152/jmi.53.229.
- Kochi, I., E. Takei, R. Maeda, K. Ito, J. Magara, T. Tsujimura, S. Kulvanich, and M. Inoue. 2021. Changes of bolus properties and the triggering of swallowing in healthy humans. Journal of oral rehabilitation 48 (5):592–600. doi: 10.1111/joor.13151.
- Kohyama, K., H. Sawada, M. Nonaka, C. Kobori, F. Hayakawa, and T. Sasaki. 2007. Textural evaluation of rice cake by chewing and swallowing measurements on human subjects. Bioscience, Biotechnology, and biochemistry 71 (2):358–65. doi: 10.1271/bbb.60276.
- Koike, S., T. Sujino, H. Ohmori, K. Shimazaki, E. Fukuyama, T. Kanai, T. Hibi, and T. Ono. 2013. Gastric emptying rate in subjects with malocclusion examined by [(13) C] breath test. Journal of oral rehabilitation 40 (8):574–81. doi: 10.1111/joor.12073.
- Kokkinos, A., C. W. le Roux, K. Alexiadou, N. Tentolouris, R. P. Vincent, D. Kyriaki, D. Perrea, M. A. Ghatei, S. R. Bloom, N. Katsilambros, et al. 2010. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon-like peptide-1. The Journal of clinical endocrinology and metabolism 95 (1):333–7. doi: 10.1210/jc.2009-1018.
- Kong, K. A., J. Park, S-h. Hong, Y. S. Hong, Y.-A. Sung, and H. Lee. 2017. Associations between body mass index and mortality or cardiovascular events in a general Korean population. PLoS One 12 (9):e0185024. doi: 10.1371/journal.pone.0185024.
- Kwon, S. H., H. R. Park, Y. M. Lee, S. Y. Kwon, O. S. Kim, H. Y. Kim, and Y. S. Lim. 2017. Difference in food and nutrient intakes in Korean elderly people according to chewing difficulty: Using data from the Korea National Health and Nutrition Examination Survey 2013 (6th). Nutrition Research and Practice 11 (2):139–46. doi: 10.4162/nrp.2017.11.2.139.
- Leischker, A. H., G. F. Kolb, and S. Felschen-Ludwig. 2010. Nutritional status, chewing function and vitamin deficiency in geriatric inpatients. European Geriatric Medicine 1 (4):207–12. doi: 10.1016/j.eurger.2010.06.006.
- Li, J., N. Zhang, L. Hu, Z. Li, R. Li, C. Li, and S. Wang. 2011. Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young Chinese men. The American Journal of clinical nutrition 94 (3):709–16. doi: 10.3945/ajcn.111.015164.
- Liedberg, B., K. Stoltze, P. Norlén, and B. Owall. 2007. Inadequate’ dietary habits and mastication in elderly men. Gerodontology 24 (1):41–6. doi: 10.1111/j.1741-2358.2007.00150.x.
- Liedberg, B., P. Norlén, B. Owall, and K. Stoltze. 2004. Masticatory and nutritional aspects on fixed and removable partial dentures. Clinical Oral Investigations 8 (1):11–7. doi: 10.1007/s00784-003-0223-6.
- Lucas, P. W., and D. A. Luke. 1986. Is food particle size a criterion for the initiation of swallowing? Journal of oral rehabilitation 13 (2):127–36. doi: 10.1111/j.1365-2842.1986.tb00645.x.
- Lund, J. P. 1991. Mastication and its control by the brain stem. Critical Reviews in Oral Biology and Medicine 2 (1):33–64. doi: 10.1177/10454411910020010401.
- Madhu, V., A. Shirali, P. N. Pawaskar, D. Madi, N. Chowta, and J. T. Ramapuram. 2016. Mastication frequency and postprandial blood sugar levels in normoglycaemic and dysglycaemic individuals: A cross-sectional comparative study. Journal of Clinical and Diagnostic Research 10 (7):Oc06–8. doi: 10.7860/JCDR/2016/18855.8082.
- Maeda, R., E. Takei, K. Ito, J. Magara, T. Tsujimura, and M. Inoue. 2020. Inter-individual variation of bolus properties in triggering swallowing during chewing in healthy humans. Journal of oral rehabilitation 47 (9):1161–70. doi: 10.1111/joor.13044.
- Magara, J., W. Onuki, R. Ita, T. Tsujimura, and M. Inoue. 2022. Chewing modulates the human cortical swallowing motor pathways. Physiology & Behavior 249:113763. doi: 10.1016/j.physbeh.2022.113763.
- Mann, T., R. Heuberger, and H. Wong. 2013. The association between chewing and swallowing difficulties and nutritional status in older adults. Australian Dental Journal 58 (2):200–6. doi: 10.1111/adj.12064.
- Marshall, T. A., J. J. Warren, J. S. Hand, X. J. Xie, and P. J. Stumbo. 2002. Oral health, nutrient intake and dietary quality in the very old. Journal of the American Dental Association (1939) 133 (10):1369–79. doi: 10.14219/jada.archive.2002.0052.
- Matsuno, K., K. Nohara, H. Fukatsu, N. Tanaka, N. Fujii, Y. Sasao, and T. Sakai. 2017. Videoendoscopic evaluation of food bolus preparation: A comparison between normal adult dentates and older adult dentates. Geriatrics & Gerontology International 17 (2):226–31. doi: 10.1111/ggi.12697.
- Medeiros, M. M. D., M. A. Pinheiro, O. M. C. Figueredo, L. F. S. Oliveira, R. L. Wanderley, Y. W. Cavalcanti, and R. C. M. Rodrigues Garcia. 2020. Masticatory function in nursing home residents: Correlation with the nutritional status and oral health–related quality of life. Journal of Oral Rehabilitation 47 (12):1511–20. doi: 10.1111/joor.13096.
- Mercier, P., and P. Poitras. 1992. Gastrointestinal symptoms and masticatory dysfunction. Journal of gastroenterology and hepatology 7 (1):61–5. doi: 10.1111/j.1440-1746.1992.tb00937.x.
- Mioche, L., P. Bourdiol, and M.-A. Peyron. 2004. Influence of age on mastication: Effects on eating behaviour. Nutrition Research Reviews 17 (1):43–54. doi: 10.1079/NRR200375.
- Mioche, L., P. Bourdiol, and S. Monier. 2003. Chewing behaviour and bolus formation during mastication of meat with different textures. Archives of oral biology 48 (3):193–200. doi: 10.1016/S0003-9969(03)00002-5.
- Miquel, S., M. Aspiras, and J. E. L. Day. 2018. Does reduced mastication influence cognitive and systemic health during aging? Physiology & Behavior 188:239–50. doi: 10.1016/j.physbeh.2018.02.018.
- Mishellany, A., A. Woda, R. Labas, and M.-A. Peyron. 2006. The challenge of mastication: Preparing a bolus suitable for deglutition. Dysphagia 21 (2):87–94. doi: 10.1007/s00455-006-9014-y.
- Monaco, A., R. Cattaneo, C. Masci, A. Spadaro, and G. Marzo. 2012. Effect of ill-fitting dentures on the swallowing duration in patients using polygraphy. Gerodontology 29 (2):e637-44–e644. doi: 10.1111/j.1741-2358.2011.00536.x.
- Motokawa, K., Y. Mikami, M. Shirobe, A. Edahiro, Y. Ohara, M. Iwasaki, Y. Watanabe, H. Kawai, T. Kera, S. Obuchi, et al. 2021. Relationship between chewing ability and nutritional status in Japanese older adults: A cross-sectional study. International Journal of Environmental Research and Public Health 18 (3):1216. doi: 10.3390/ijerph18031216.
- Müller, F., E. Duvernay, A. Loup, L. Vazquez, F. R. Herrmann, and M. Schimmel. 2013. Implant-supported mandibular overdentures in very old adults: A randomized controlled trial. Journal of dental research 92 (12 Suppl):154S–60S. doi: 10.1177/0022034513509630.
- Nakata, M. 1998. Masticatory function and its effects on general health. International Dental Journal 48 (6):540–8. doi: 10.1111/j.1875-595x.1998.tb00489.x.
- Nishi, T., M. Ohta, T. Takano, K. Ogami, T. Ueda, and K. Sakurai. 2022. Oral function is associated with the body and muscle mass indices of middle-aged dental patients. Clinical and experimental dental research 8 (1):217–24. doi: 10.1002/cre2.514.
- Nuttall, F. Q. 2015. Body mass index: Obesity, BMI, and health: A critical review. Nutrition Today 50 (3):117–28. doi: 10.1097/NT.0000000000000092.
- Ohta, M., Y. Imamura, N. Chebib, R. M. Schulte‐Eickhoff, S. Allain, L. Genton, P. Mojon, C. Graf, T. Ueda, F. Müller, et al. 2022. Oral function and nutritional status in non-acute hospitalised elders. Gerodontology 39 (1):74–82. doi: 10.1111/ger.12612.
- Okada, K., H. Enoki, S. Izawa, A. Iguchi, and M. Kuzuya. 2010. Association between masticatory performance and anthropometric measurements and nutritional status in the elderly. Geriatrics & Gerontology International 10 (1):56–63. doi: 10.1111/j.1447-0594.2009.00560.x.
- Okubo, H., K. Murakami, S. Masayasu, and S. Sasaki. 2018. The relationship of eating rate and degree of chewing to body weight status among preschool children in japan: A nationwide cross-sectional study. Nutrients 11 (1):64. doi: 10.3390/nu11010064.
- Ono, T., I. Kumakura, M. Arimoto, K. Hori, J. Dong, H. Iwata, T. Nokubi, K. Tsuga, and Y. Akagawa. 2007. Influence of bite force and tongue pressure on oro-pharyngeal residue in the elderly. Gerodontology 24 (3):143–50. doi: 10.1111/j.1741-2358.2007.00172.x.
- Ono, Y., T. Yamamoto, K. Y. Kubo, and M. Onozuka. 2010. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. Journal of oral rehabilitation 37 (8):624–40.
- Onuki, W., J. Magara, T. Tsujimura, K. Ito, H. Sakai, S. Kulvanich, Y. Nakajima, N. Saka, and M. Inoue. 2021. Survey of oral hypofunction in older outpatients at a dental hospital. Journal of oral rehabilitation 48 (10):1173–82. doi: 10.1111/joor.13237.
- Österberg, T., K. Tsuga, E. Rothenberg, G. E. Carlsson, and B. Steen. 2002. Masticatory ability in 80‐year‐old subjects and its relation to intake of energy, nutrients and food items. Gerodontology 19 (2):95–101. doi: 10.1111/j.1741-2358.2002.00095.x.
- Ouzzani, M., H. Hammady, Z. Fedorowicz, and A. Elmagarmid. 2016. Rayyan-a web and mobile app for systematic reviews. Systematic Reviews 5 (1):210. doi: 10.1186/s13643-016-0384-4.
- Page, M. J., J. E. McKenzie, P. M. Bossuyt, I. Boutron, T. C. Hoffmann, C. D. Mulrow, L. Shamseer, J. M. Tetzlaff, E. A. Akl, S. E. Brennan, et al. 2021. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clinical Research ed.) 372:n71. doi: 10.1136/bmj.n71.
- Paphangkorakit, J., K. Kanpittaya, N. Pawanja, and W. Pitiphat. 2019. Effect of chewing rate on meal intake. European Journal of oral sciences 127 (1):40–4. doi: 10.1111/eos.12583.
- Pedroni-Pereira, A., M. C. S. Marquezin, D. S. Araujo, L. J. Pereira, S. Bommarito, and P. M. Castelo. 2018. Lack of agreement between objective and subjective measures in the evaluation of masticatory function: A preliminary study. Physiology & Behavior 184:220–5. doi: 10.1016/j.physbeh.2017.12.001.
- Pennings, B., B. B. Groen, J.-W. van Dijk, A. de Lange, A. Kiskini, M. Kuklinski, J. M. Senden, and L. J. van Loon. 2013. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. The American Journal of Clinical Nutrition 98 (1):121–8. doi: 10.3945/ajcn.112.051201.
- Pera, P., C. Bucca, P. Borro, C. Bernocco, A. De Lillo, and S. Carossa. 2002. Influence of mastication on gastric emptying. Journal of Dental Research 81 (3):179–81. doi: 10.1177/0810179.
- Prinz, J. F., and P. W. Lucas. 1995. Swallow thresholds in human mastication. Archives of oral biology 40 (5):401–3. doi: 10.1016/0003-9969(94)00185-e.
- Prinz, J. F., and P. W. Lucas. 1997. An optimization model for mastication and swallowing in mammals. Proceedings. Biological Sciences 264 (1389):1715–21. doi: 10.1098/rspb.1997.0238.
- Ranawana, V. V., C. J. K. Henry, and a M. Pratt Meg. 2010. Degree of habitual mastication seems to contribute to interindividual variations in the glycemic response to rice but not to spaghetti. Nutrition Research. 30 (6):382–91. doi: 10.1016/j.nutres.2010.06.002.
- Ranawana, V., J. A. Monro, S. Mishra, and C. J. Henry. 2010. Degree of particle size breakdown during mastication may be a possible cause of interindividual glycemic variability. Nutrition Research (New York, N.Y.) 30 (4):246–‐54. doi: 10.1016/j.nutres.2010.02.004.
- Saitoh, E., S. Shibata, K. Matsuo, M. Baba, W. Fujii, and J. B. Palmer. 2007. Chewing and food consistency: Effects on bolus transport and swallow initiation. Dysphagia 22 (2):100–7. doi: 10.1007/s00455-006-9060-5.
- Saksono, P., M. Hijryana, A. Walls, L. Kusdhany, M. Indrasari, and N. Ariani. 2019. Relationships Between Tooth Loss and Masticatory Performance, Nutrition Intake, and Nutritional Status in the Elderly. Pesquisa Brasileira em Odontopediatria e Clínica Integrada 19 (1):1–8. doi: 10.4034/PBOCI.2019.191.148.
- Sanchez-Ayala, A., N. H. Campanha, and R. C. M. R. Garcia. 2013. Relationship between body fat and masticatory function. Journal of Prosthodontics 22 (2):120–5. doi: 10.1111/j.1532-849X.2012.00937.x.
- Sawada, N., N. Takeuchi, D. Ekuni, and M. Morita. 2021. Oral function, nutritional status and physical status in Japanese independent older adults. Gerodontology. doi: 10.1111/ger.12593.
- Sheiham, A., and J. Steele. 2001. Does the condition of the mouth and teeth affect the ability to eat certain foods, nutrient and dietary intake and nutritional status amongst older people? Public Health Nutrition 4 (3):797–803. doi: 10.1079/phn2000116.
- Sierpinska, T., M. Golebiewska, J. W. Dlugosz, A. Kemona, and W. Laszewicz. 2007. Connection between masticatory efficiency and pathomorphologic changes in gastric mucosa. Quintessence International (Berlin, Germany : 1985) 38 (1):31–7.
- Simonian, H. P., K. M. Kresge, G. H. Boden, and H. P. Parkman. 2005. Differential effects of sham feeding and meal ingestion on ghrelin and pancreatic polypeptide levels: Evidence for vagal efferent stimulation mediating ghrelin release. Neurogastroenterology and Motility 17 (3):348–54. doi: 10.1111/j.1365-2982.2004.00634.x.
- Slagter, A. P., L. W. Olthoff, F. Bosman, and W. H. Steen. 1992. Masticatory ability, denture quality, and oral conditions in edentulous subjects. The Journal of prosthetic dentistry 68 (2):299–307. doi: 10.1016/0022-3913(92)90334-7.
- Son, D.-S., J. W. Seong, Y. Kim, Y. Chee, and C. H. Hwang. 2013. The effects of removable denture on swallowing. Annals of rehabilitation medicine 37 (2):247–53. doi: 10.5535/arm.2013.37.2.247.
- Sumonsiri, P., U. Thongudomporn, and J. Paphangkorakit. 2018. Correlation between the median particle size of chewed frankfurter sausage and almonds during masticatory performance test. Journal of Oral Rehabilitation 45 (7):512–7. doi: 10.1111/joor.12639.
- Sumonsiri, P., U. Thongudomporn, J. Paphangkorakit, and T. Premprabha. 2019. Assessment of the relationship between masticatory performance, occlusal contact area, chewing time and cycles, and gastric emptying scintigraphy in dentate subjects. Journal of oral rehabilitation 46 (9):787–91. doi: 10.1111/joor.12812.
- Suzuki, H., M. Fukushima, S. Okamoto, O. Takahashi, T. Shimbo, T. Kurose, Y. Yamada, N. Inagaki, Y. Seino, and T. Fukui. 2005. Effects of thorough mastication on postprandial plasma glucose concentrations in nonobese Japanese subjects. Metabolism 54 (12):1593–9. doi: 10.1016/j.metabol.2005.06.006.
- Tada, A., and H. Miura. 2018. Association of mastication and factors affecting masticatory function with obesity in adults: A systematic review. BMC Oral Health 18 (1):76. doi: 10.1186/s12903-018-0525-3.
- Takeshima, T., Y. Fujita, and K. Maki. 2019. Factors associated with masticatory performance and swallowing threshold according to dental formula development. Archives of oral biology 99:51–7. doi: 10.1016/j.archoralbio.2018.12.012.
- Tosello, A., B. Foti, C. Sédarat, J. M. Brodeur, J. M. Ferrigno, P. Tavitian, G. Susini, and J. J. Bonfil. 2001. Oral functional characteristics and gastrointestinal pathology: An epidemiological approach. Journal of oral rehabilitation 28 (7):668–72. doi: 10.1046/j.1365-2842.2001.00730.x.
- van der Bilt, A. 2011. Assessment of mastication with implications for oral rehabilitation: A review. Journal of oral rehabilitation 38 (10):754–80. doi: 10.1111/j.1365-2842.2010.02197.x.
- Wada, S., N. Kawate, and M. Mizuma. 2017. What type of food can older adults masticate?: Evaluation of mastication performance using color-changeable chewing gum. Dysphagia 32 (5):636–43. doi: 10.1007/s00455-017-9807-1.
- Wada, S., T. Goto, K. Fujimoto, M. Watanabe, K. Nagao, A. Nakamichi, and T. Ichikawa. 2017. Changes in food bolus texture during mastication. Journal of texture studies 48 (2):171–7. doi: 10.1111/jtxs.12228.
- Wallace, S. M. G. McKenna, and M. Schimmel. 2017. Impact of prosthodontic rehabilitation on the masticatory performance of partially dentate older patients: Can it predict nutritional state? Results from a RCT. Proceedings of the nutrition society Conference: nutrition society irish section conference 2017: What governs what we eat? United kingdom, 76(OCE3):E109. doi: 10.1017/S0029665117001823.
- Wallace, S., S. Samietz, M. Abbas, G. McKenna, J. V. Woodside, and M. Schimmel. 2018. Impact of prosthodontic rehabilitation on the masticatory performance of partially dentate older patients: Can it predict nutritional state? Results from a RCT. Journal of Dentistry 68:66–71. doi: 10.1016/j.jdent.2017.11.003.
- Weijenberg, R. A. F., S. Delwel, B. V. Ho, C. D. van der Maarel-Wierink, and F. Lobbezoo. 2019. Mind your teeth—The relationship between mastication and cognition. Gerodontology 36 (1):2–7. doi: 10.1111/ger.12380.
- Weijenberg, R. A., E. J. Scherder, and F. Lobbezoo. 2011. Mastication for the mind–the relationship between mastication and cognition in ageing and dementia. Neuroscience and biobehavioral reviews 35 (3):483–97. doi: 10.1016/j.neubiorev.2010.06.002.
- White, A. K., B. Venn, L. W. Lu, E. Rush, L. M. Gallo, J. L. C. Yong, and M. Farella. 2015. A comparison of chewing rate between overweight and normal BMI individuals. Physiology & Behavior 145:8–13. doi: 10.1016/j.physbeh.2015.03.028.
- Wöstmann, B., K. Michel, B. Brinkert, A. Melchheier-Weskott, P. Rehmann, and M. Balkenhol. 2008. Influence of denture improvement on the nutritional status and quality of life of geriatric patients. Journal of dentistry 36 (10):816–21. doi: 10.1016/j.jdent.2008.05.017.
- Yamamoto, H., J. Furuya, Y. Tamada, and H. Kondo. 2013. Impacts of wearing complete dentures on bolus transport during feeding in elderly edentulous. Journal of Oral Rehabilitation 40 (12):923–31. doi: 10.1111/joor.12107.
- Yamashita, S., D. Sugita, and K. Matsuo. 2013. Relationship between stage II transport and number of chewing strokes as mastication progresses. Physiology & Behavior 122:100–3. doi: 10.1016/j.physbeh.2013.08.030.
- Yellowitz, J. A. 2016. Building the Ideal Interdisciplinary Team to Address Oral Health. Generations: Journal of the American Society on Aging 40 (3):60–5.
- Zhu, Y., and J. H. Hollis. 2014a. Chewing thoroughly reduces eating rate and postprandial food palatability but does not influence meal size in older adults. Physiology & Behavior 123:62–6. doi: 10.1016/j.physbeh.2013.10.003.
- Zhu, Y., and J. H. Hollis. 2014b. Increasing the number of chews before swallowing reduces meal size in normal-weight, overweight, and obese adults. Journal of the Academy of Nutrition and Dietetics 114 (6):926–31. doi: 10.1016/j.jand.2013.08.020.
- Zhu, Y., and J. H. Hollis. 2015a. Differences in chewing behaviors between healthy fully dentate young and older adults assessed by electromyographic recordings. International Journal of food sciences and nutrition 66 (4):452–7. doi: 10.3109/09637486.2015.1038222.
- Zhu, Y., and J. H. Hollis. 2015b. Relationship between chewing behavior and body weight status in fully dentate healthy adults. International Journal of Food Science and Nutrition. 66 (2):135–9. doi: 10.3109/09637486.2014.979317.
- Zhu, Y., W. H. Hsu, and J. H. Hollis. 2013. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. British Journal of Nutrition 110 (2):384–90. doi: 10.1017/S0007114512005053.
- Zhu, Y., W. H. Hsu, and J. H. Hollis. 2014. Increased number of chews during a fixed-amount meal suppresses postprandial appetite and modulates glycemic response in older males. Physiology & Behavior 133:136–40. doi: 10.1016/j.physbeh.2014.05.011.