Abstract
The incidence of diabetes mellitus is dramatically increasing every year, causing a huge global burden. Moreover, existing anti-diabetic drugs inevitably bring adverse reactions, and the application of islet transplantation is often limited by the damage caused by oxidative stress after transplantation. Thus, new approaches are needed to combat the growing burden of diabetes mellitus. Anthocyanins are of great nutritional interest and have been documented that have beneficial effects on chronic diseases, including diabetes mellitus. Here, we describe the health effects of anthocyanins on diabetes mellitus and islet transplantation. Epidemiological studies demonstrated that moderate intake of anthocyanins leading to a reduction in risk of diabetes mellitus. Numerous experiments both animal and clinical studies also showed positive effects of anthocyanins on prevention and treatment of diabetes and diabetic complications. These effects of anthocyanins may be related to mechanisms of improving glucose and lipid metabolism and insulin resistance, antioxidant, and anti-inflammatory activities. In addition, damage and function of pancreatic islets after transplantation are also improved by anthocyanins. These findings suggest that daily intake of anthocyanins may not only improve nutritional metabolism in healthy individuals to prevent from diabetes, but also as a supplementary treatment of diabetes mellitus and islet transplantation. Thus, more evidence is needed to better understand the potential health benefits of anthocyanins.
Introduction
Diabetes mellitus (DM), a group of metabolic diseases, is characterized by hyperglycemia resulting from reduces in islet β-cell numbers or defects in islet β-cell function, and as hyperglycemia continues to progress and rapid swings, it can affect nearly every tissue of body and lead to severe acute and chronic diabetes complications (Chellappan et al. Citation2018). DM has reached warning line across the globe, and approximately 463.0 million individuals are affected by DM in 2019. It is estimated that the number of patients will reach 578 million in 2030 and 700 million in 2045 (Saeedi et al. Citation2019). DM are mainly divided into various types by different causes, type 1 diabetes mellitus (T1DM), type 2 diabetes mellitus (T2DM), genetic defects of the β-cell function and other specific types of diabetes (Petersmann et al. Citation2019).
T1DM, an autoimmune disorder, accounting for approximately 5% of diabetic patients, is mainly due to the increase of the expression of surface leucocyte-homing receptors in the early endothelial cells, which causes immune cells to enter the endothelial tissue, leading to the destruction of β-cells (Eizirik, Pasquali, and Cnop Citation2020). T2DM, a metabolic disease with a strong genetic susceptibility, accounting for about 90% of all diabetic patients, is mainly due to insulin resistance or relative insulin deficiency without islet β-cell destruction (Mayans Citation2015). As insulin sensitivity progressively deteriorates, pancreatic cells increase insulin secretion leading to hyperinsulinemia, and as this coordination mechanism is out of balance, ultimately leading to β-cell dysfunction. There are different treatment principles between T1DM and T2DM. As for T1DM, primary treatment modality is exogenous insulin replacement therapy, while insulin therapy is used only after other anti-diabetic drugs cannot control hyperglycemia in T2DM (Lu and Zhao Citation2020). Furthermore, both T1DM and T2DM can induce a series of complications, including acute and chronic complications. Acute complications, including diabetic ketoacidosis and hyperosmolar hyperglycemia nonketotic coma, usually result from inadequate treatment. Chronic complications can be divided into microangiopathy and macroangiopathy, the former mainly includes diabetic retinopathy (DR), diabetic nephropathy (DN), and diabetic neuropathy, the latter mainly occurs in the coronary and cerebrovascular arteries (Cole and Florez Citation2020). DM and related complications have generated huge global burdens and become a global health problem.
Pancreatic islet transplantation, one of the safest and least invasive transplant procedures, has become as a reliable treatment for insulin-deficient diabetes(Shapiro, Pokrywczynska, and Ricordi Citation2017, Rickels and Robertson Citation2019). The therapeutic effect of islet transplantation depends on the numbers of the functional islet β-cells surviving after transplantation(Rickels and Robertson Citation2019). However, islets are damaged or even die due to ischemia-reperfusion and oxidative stress during islet isolation and following transplantation. Because of lacking an intrinsic vascular system, islets are rather vulnerable to ischemia-reperfusion injury which generated reactive oxygen species (ROS) further leading to the loss of islet β-cells(Miki et al. Citation2018). In addition, in the late stage of transplantation, the islet graft underwent immune reject reaction and gradually lose function due to oxidative stress and subsequent immune response(Montanari et al. Citation2019). Therefore, how to maintain the long-term activity and function of transplanted islets is a critical issue for its successful clinical application.
COVID-19, an ongoing pandemic, sweeping the world. Many diabetic patients with COVID-19 are insensitive to existing hypoglycemic drugs, and are even impaired by the side effects of the drugs(Panchamoorthy and Vel Citation2021). Thus, a growing body of research is focusing on novel natural products possessing anti-DM effect, such as medicine plant(Salehi et al. Citation2019, Ashande et al. Citation2022) and fresh fruit containing phenolic(Mhya, Nuhu, and Mankilik Citation2021) and flavonoids compounds(Al-Ishaq et al. Citation2019). Anthocyanins, natural plant flavonoid pigment, providing many fruits and flowers with various colors, which often provide benefits over other phytochemicals, have gathered widespread attention in recent decades (Fang Citation2014, Kalt et al. Citation2020). Due to unique properties and various effects, anthocyanins are widely used in a variety of industries, including food processing industry, clean energy industry, and pharmaceutical industry(Buchweitz et al. Citation2013, Chien and Hsu Citation2013, Alappat and Alappat Citation2020). The health benefits of anthocyanins are mainly associated with antioxidant, anti-inflammatory, anti-cancer, and gut microbiota modulation(Tian et al. Citation2019). Moreover, anthocyanins also show glucose regulation, and neuroprotective effects(Mattioli et al. Citation2020). At present, these effects have been proven to be effective in treating or preventing chronic diseases in studies, such as cardiovascular disease (CVD)(Wang et al. Citation2014), neurodegenerative diseases(Winter and Bickford Citation2019), vision loss disease(Nomi, Iwasaki-Kurashige, and Matsumoto Citation2019), tumor(Lin et al. Citation2017), and DM(Guo et al. Citation2016). Many features of DM, such as hyperglycemia, insulin deficiency, and insulin resistance, can be ameliorated by increasing consumption of anthocyanins(Cao et al. Citation2019). It is an appealing direction exploring the health benefits of anthocyanins for DM and islet transplantation, hence research on the role of anthocyanins in prevention and treatment of DM (including associated complications), functional preservation of islet grafts in islet transplantation is reviewed in this narrative, focusing on animal and human studies, as well as possible mechanism. Over nearly 150 references cited in this article were published in the recent ten years.
Current Status of knowledge
Chemical structure and bioavailability of anthocyanins
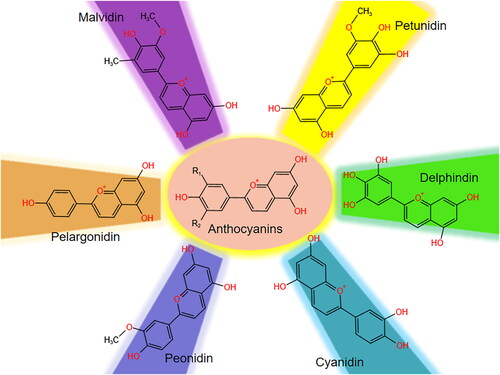
In fresh fruits and vegetables, anthocyanins can be divided into six common types, such as cyanidin, peonidin, pelargonidin, malvidin, delphinidin, and petunidin, based on the different substituent groups on the flavylium B‐ring(Millar, Duclos, and Blesso Citation2017) (). They are similar molecules, including a benzoic ring(A ring), a non-benzoic ring with an oxygen atom inside(C ring), and another benzoic ring with a carbon–carbon bound (C–C) named as flavylium ion(B ring)(Andersen et al. Citation2010). In addition to providing various colors, these compounds play significant roles in the process of plant growth and development, including reproduction and defense mechanisms(Alappat and Alappat Citation2020). The main way to obtain anthocyanins is to extract and separate from plant tissues, such as blueberries, bayberries, and strawberries(Silva et al. Citation2017). Previously, it was difficult to associate anthocyanins with health outcomes, mainly due to their most striking features that quickly absorbed and excreted, but low in absorption efficiency, which means that the concentration of anthocyanins in plasma rarely reaches therapeutic level (Manach et al. Citation2005). Meanwhile, the stability of anthocyanins is poor and susceptible to temperature, pH, and light (Yang et al. Citation2018). The bioavailability of anthocyanins has been shown to be totally underestimated resulted from quickly absorbed and transformed into biologically active metabolites. And metabolized conjugates of these full sets of anthocyanins have not yet been completely determined(Lila et al. Citation2016). Meanwhile, to enhance the biological activity of anthocyanins, many advanced approaches have been designed, such as using lipid-, polysaccharide- and protein-based complexes, nanocarriers and exosome vehicles(Shen et al. Citation2022).
Relation of anthocyanins’ health effects to its structure
Anthocyanins possess antioxidant and anti-cancer abilities closely related to its unique chemical structure. The benzene ring skeleton provides anthocyanins with special function ensuring the stability of the structure under the disappearance of electrons. The antioxidant efficacy of anthocyanins is greatly dependent on their chemical structure, such as the number and position of hydroxyl groups, the 2, 3 double bond in conjugation, the degree of glycosylation and 4-oxofunction in C ring(Reis et al. Citation2016). Due to the hydroxyl groups in the B-ring and the oxonium ion in the c-ring, anthocyanins may have two antioxidant mechanisms: hydrogen atom transfer (HAT) and single electron transfer (SET). In the HAT mechanism, antioxidants Convert free radicals into stable products by donating a hydrogen atom. For the SET mechanism, antioxidants maintain stability by providing free radical electrons(Liang and Kitts Citation2014). The anticancer effect of anthocyanins is closely related to the substituents on the B ring. With the different substituents on the B ring, the anticancer effects are also different(Lin et al. Citation2017). Especially the ortho-dihydroxyphenyl structure on the B-ring showed the strongest anticancer effect, preventing tumor metastasis and growth (Hou et al. Citation2004, Xu et al. Citation2010).
In addition, anthocyanins play anti-inflammatory effect through multiple mechanisms: inhibiting the activation of nuclear factor-kB (NF-kB) pathway, which is a central orchestrator of the inflammatory response(Duarte et al. Citation2018); inhibiting inducible nitric oxide synthase(iNOS) to reduce the production of nitric oxide that promotes inflammation(Ma et al. Citation2021); increasing antioxidant capacity to reduce the inflammatory reaction(Huang et al. Citation2018); inhibiting cyclooxygenase activity to decrease the release of Prostaglandin E2 leading to acute inflammation(He, Hu, et al. Citation2017). And recent evidence suggests that anthocyanins may play a health-promoting role by modulating gut microbiota(Li, Wang, et al. Citation2017). Although the stomach and small intestine can absorb anthocyanins, more than half of the anthocyanins eventually reach the colon, and then play a prebiotic active role under the interaction with colonic microorganisms(He and Giusti Citation2010, Faria et al. Citation2014). Gathering information from in vitro and human studies, proves that anthocyanins have an influence on intestinal bacterial growth, especially beneficial bacteria, such as Bifidobaterium spp.and Lactobacillus-Enterococcus(Hidalgo et al. Citation2012, Boto-Ordóñez et al. Citation2014, Sun et al. Citation2018, Zhu, Sun et al. Citation2018).
Anthocyanins and prevention of diabetes mellitus
Pre-diabetes is the term that defines some people who with impaired glucose tolerance but do not meet the diagnostic criteria for diabetes and eventually develop DM(Beulens et al. Citation2019, Khan et al. Citation2019). In a simulation model, effective control of blood glucose can delay the progression of DM in patients with prediabetes(DeJesus et al. Citation2017). In addition, obesity was an independent predictor for pre-diabetes progression to DM, and with increasing BMI, the risk of progression to DM progressively increased(Ligthart et al. Citation2016).
Animal studies
Several animal studies have also been evaluated preventive effect of anthocyanins on DM (). In an animal study, after the rats with prediabetes and hyperlipidemia were administered black chokeberry fruit extract that contains high amounts of anthocyanins, antioxidant status was increased, and blood lipids and blood glucose was decreased(Jurgoński, Juśkiewicz, and Zduńczyk Citation2008). Insulin resistance in mice with high fat diet were improved after 12 weeks of diet supplementation with cyanidin 3-glucoside-rich purple corn color(Tsuda et al. Citation2003). C57BL/6 mice with obese and hyperglycemic fed a high fat diet containing blueberry anthocyanins for 13 weeks had better glucose tolerance and fasting blood glucose levels, and lower serum cholesterol than mice fed a high fat diet without anthocyanins. In addition, blueberry anthocyanins significantly reduced glucose production in rat hepatocytes to improve blood glucose levels but did not increase glucose uptake by L6 myotubes(Roopchand et al. Citation2013).
Table 1. the anti-T2DM effect of anthocyanins in clinical trial.
Animal models fed a high-fat diet often develop obesity, insulin resistance, impaired glucose tolerance, and eventually DM(Heydemann Citation2016). Anthocyanins can improve these manifestations and thus play a role in preventing DM. Tian and colleagues reportedcyanidin-3-glucoside(C3G) reduced body-weight gain and improved glucose tolerance in mice fed a high-fat diet(Tian et al. Citation2020). Purple sweet potato extract induces adipocyte browning by increasing expression of adipose browning-related genes in high-fat-diet-induced obese mice, which means converting stored adipocytes into heat-producing adipocytes to improve lipid deposition(Lee et al. Citation2021). C57BL/6 mice fed a high-fat (45%) diet plus anthocyanin-rich tart cherry extract for 8 weeks had lower the leptin and IL-6 levels and higher adiponectin concentration, compared to controls group only fed by high-fat diet(Nemes et al. Citation2019). Blueberries, a rich source of anthocyanins, have been shown in multiple studies to reduce body weight, improve lipid profiles, down-regulate the expression of inflammatory factors, and improve insulin sensitivity in high-fat diet-fed animal models(Seymour et al. Citation2011, Wu, Jiang, et al. Citation2016, Lee et al. Citation2018). Black current that has a variety of anthocyanins have been reported to significantly inhibit the elevation of cholesterol and triglycerides, and thereby reduce hepatic triglyceride levels by reducing the expression of genes related to fatty acid synthesis in ovariectomized rats(Park et al. Citation2015). In addition, black currant has been shown to improve Lipid metabolism by regulating the expression of genes related to the synthesis and degradation of lipids and cholesterols in other animal experiments(Song, Shen, Wang, et al. Citation2021). Other anthocyanin-rich black rice(Song, Shen, Wang, et al. Citation2021), blackelderberry(Farrell et al. Citation2015),fruit of acanthopanax senticosus(Saito et al. Citation2016),aronia melanocarpa(Kim et al. Citation2018, Lim et al. Citation2019), andraspberry(Wu et al. Citation2018) improved lipid metabolism in liver or serum and insulin resistance in diet-induced obese mice.
Clinical trial
In a cohort study, 60586 women with T2DM follow for 20 years demonstrated the importance of anthocyanin-rich foods, which can prevent T2DM risk(Laouali et al. Citation2020). A dose-response manner between anthocyanin intake and T2DM risk was demonstrated (HR: 0.86; 95% CI: 0.81, 0.91) in two meta-analysis studies, with increased intake of anthocyanins leading to T2DM risk reduction(Rienks et al. Citation2018, Xu et al. Citation2018). In a study that data from 3 prospective cohort studies in US proved that the risk of developing T2DM was significantly reduced after a high intake of anthocyanins (RR = 0.85; 95% CI: 0.80, 0.91). Furthermore, the increased consumption of anthocyanin -rich foods, such as blueberries, apples, and pears, also decrease T2DM risk(Wedick et al. Citation2012, Palacios, Kramer, and Maki Citation2019). In its subsequent study, it was further demonstrated that consuming fruit juice 3 times a week, which contains high levels of anthocyanins, reduces the risk of T2DM by 14%-33%(Muraki et al. Citation2013). Similar results were also demonstrated when was determined in a Polish cohort (RR: 0.68, 95% CI: 0.48–0.98)(Grosso et al. Citation2017).A dose–response meta-analyses suggests that a daily intake of 7.5 mg increment of dietary anthocyanins or 17 g of berries per day reduces T2DM risk by 5%(Guo et al. Citation2016). It is worth noting that not all observational studies have shown that anthocyanins are associated with the risk of T2DM(Jacques et al. Citation2013).
Many studies have demonstrated a decrease in the risk of T2DM with increased intake of anthocyanins(Wedick et al. Citation2012, Guo et al. Citation2016). Insulin resistance and insulin deficiency due to pancreatic beta-cell dysfunction are core pathophysiological properties of T2DM. In a randomized cross-over trial, obese subjects had lower serum insulin area under the curve following anthocyanin-rich mixed-berry treatment, implying the potential ability of anthocyanins to improve insulin resistance(Solverson et al. Citation2019). Then, after intake of beverage containing freeze-dried whole strawberry powder by 21 adults with insulin resistance, a reduction in was documented in insulin demand after meal in obese patients, meaning insulin sensitivity of subjects was improved(Park et al. Citation2016). In a randomized controlled trial serum adipsin increased and visfatin decreased in participants with prediabetes or newly diagnosed diabetes following 12 weeks of anthocyanin supplementation. In addition, HbA1c, apolipoprotein A-1 and apolipoprotein B were also significantly improved(Yang et al. Citation2021). Similar results were observed in participants with prediabetes or early untreated DM in a randomized controlled trial in China(Yang et al. Citation2017). However, in another randomized controlled trial it was found that the improvement in blood glucose and lipids was only in patients with newly diagnosed DM, not in patients with prediabetes(Yang et al. Citation2020). In 36 prediabetic subjects, fasting blood glucose and insulin levels was dose-dependently reduced after delphinol one hour before glucose intake(Alvarado, Leschot, et al. Citation2016). And Hidalgo et al found that delphinol can effectively improve post prandial blood glucose and insulin in volunteers with moderate glucose intolerance(Hidalgo et al. Citation2014). But in another three-month clinical trial focusing on the effects of delphinol on prediabetic individuals, after 3 months of delphinol intake, average levels of HbA1c were significantly improved, but not fasting insulin and glucose(Alvarado, Schoenlau, et al. Citation2016). Insulin sensitivity improved after individuals with insulin resistance were treated with freeze-dried whole blueberry powder for 6 weeks(Stull et al. Citation2010).
Anthocyanins can also play a role in preventing DM by improving lipid metabolism. In a double-blind, placebo-controlled study of obese adults, lipid metabolism improved after 8 weeks of ingestion of anthocyanin-rich black soybean testa extracts(Lee et al. Citation2016). In a pilot study, 11 obese women had lower total and LDL cholesterol after a 12-week diet of commercial red orange juice(Azzini et al. Citation2017). Likewise, volunteers also experienced a drop in LDL cholesterol after consuming red orange juice daily for 8 weeks(Silveira, Dourado, and Cesar Citation2015). In clinical research on black rice extract, postmenopausal women reduced fat accumulation after oral ingestion for 12 weeks(Jung et al. Citation2021). Intake of blackberries that has rich anthocyanins for 7 days by 27 overweight or obese men was associated with an increase in fat oxidation and an improvement in insulin sensitivity(Solverson et al. Citation2018).
The result of the clinical trials discussed above about the benefits of anthocyanins on T2DM is presented in .
Anthocyanins and treatment of diabetes mellitus
Type 2 diabetes mellitus
Animal trial
demonstrates the beneficial effect anthocyanins on T2DM in animal studies. T2DM mice that consumed bilberry extract that contain large amounts of anthocyanins had remarkable reduced the blood glucose concentration and enhanced insulin sensitivity(Takikawa et al. Citation2010). Anthocyanins from maqui berry improved fasting blood glucose levels and glucose tolerance, decreased hepatic glucose production, and increased glucose uptake by L6 myotubes in hyperglycemic obese C57BL/6J mice(Rojo et al. Citation2012). In a study, T2DM mice were fed with blackcurrant extract that contain high levels of anthocyanins, blood glucose concentration decreased, and glucose tolerance greatly improved(Iizuka et al. Citation2018).Several reports also have found an improvement in blood sugar and glucose tolerance(Guo, Xia, et al. Citation2012, Kurimoto et al. Citation2013, Choi et al. Citation2016, Oliveira et al. Citation2017), but other reports have not found such protective effects of anthocyanins in animal models(Mykkänen et al. Citation2014, Oyama et al. Citation2016). T2DM mice were fed a diet containing 0%, 5%, or 10% buckwheat sprouts, and the results showed that as the concentration of buckwheat sprouts in the diet increased, blood glucose and lipids improved more obviously(Watanabe and Ayugase Citation2010). And anthocyanins from other plants have also showed properties of improving blood glucose and lipid(Liu et al. Citation2020). Chen et al also found that anthocyanins extracted from black soybean seed coat could improve hyperglycemia and hyperlipidemia of STZ-induced T2DM mice and reduce the damage of liver, kidney, and pancreas(Chen et al. Citation2018). In other animal study, treatment of T2DM mice with blueberry anthocyanin extract improved blood glucose levels and glucose tolerance, relieved symptoms of polydipsia and polyuria, and decreased TC, TG, and insulin levels(Herrera-Balandrano et al. Citation2021). Diabetes model KK-Ay mice that consumed aronia juice had reduced body weights, blood glucose levels, and the weights of white adipose tissues, compared with the control group(Yamane et al. Citation2016). In addition to improving blood glucose and lipids level, anthocyanins from purple sweet potato were proved to decrease oxidative stress and liver damage in STZ-induced T2DM rats(Jiang et al. Citation2020).
Table 2. Anti-T2DM of anthocyanins in animal trial.
Mulberry anthocyanin extract not only alleviated insulin resistance and lowered blood sugar in HepG2 cells in vitro studies, but also improved parameters of blood sugar and blood lipids and increased adiponectin levels in db/db mice in vitro studies(Yan, Dai, and Zheng Citation2016). Similar results were also seen in db/db mice after 6 weeks of C3G intervention(Ye et al. Citation2022). Black soybean seed coat extract and cyanidin-3-glucoside (C3G) were shown to induce adipocytes to differentiate into smaller, more insulin-sensitive adipocytes in vivo and in vitro studies(Matsukawa et al. Citation2015). Moreover, after diabetic db/db mice received dietary C3G supplementation for 5 weeks, the mice had reduced hepatic triglyceride content and steatosis, as well as decreased serum concentrations of inflammatory cytokines(Guo, Xia, et al. Citation2012). In addition, C3G can improve endothelial function and alleviate oxidative stress in pancreas islets.(Liu et al. Citation2014, Ye et al. Citation2021).
In addition, anthocyanins also have synergistic effects with conventional T2DM drugs. After combined treatment with fenofibrate and anthocyanins (from Black soybeans), the serum postprandial triglyceride level and LDL cholesterol concentration were significantly lowered in T2DM patients complicated with postprandial hyperlipidemia, compared to that with Fenofibrate alone(Kusunoki et al. Citation2015). In an animal experiment, malvidin, an anthocyanins compound, was combined with metformin to treat STZ-induced diabetic rats, and the results showed that the combination therapy had more alleviated insulin resistance, improved lipid metabolism, and reduced fasting blood glucose, serum insulin compared with single therapy(Zou et al. Citation2021). Similarly, metformin and anthocyanin exhibit significant synergistic effects in lowering blood glucose and improving insulin resistance both in vitro and in vivo(Tian et al. Citation2022).
Clinical trial
In a clinical trial, T2DM individuals after 4 weeks of ingestion of 320 mg of anthocyanins per day significantly reduced fasting blood glucose and LDL-cholesterol and some proinflammatory molecules such as IL-6, IL-18, and tumor necrosisi factor (TNF-α), increased some anti-inflammatory molecules such as IL-10 and adiponectin(Nikbakht et al. Citation2021). However, in another randomized, placebo-controlled study, short-term (4 weeks) supplementation with anthocyanins from bilberry had no significant effect on patients with T2DM. It may need longer treatment period to have beneficial effects based on trends in glycemic control(Chan et al. Citation2021). Moreover, bilberry extract has effects on glucose metabolism in T2DM, postprandial glycemia and insulin of T2DM subjects decreased after a single oral dose of bilberry extract that contains 36% (w/w) anthocyanins(Hoggard et al. Citation2013). Anthocyanins from Vaccinium Arctostaphylos extract lowered the blood levels of fasting glucose and 2-h postprandial glucose(Kianbakht, Abasi, and Dabaghian Citation2013). When some biochemical indicators in patients with T2DM after given 160 mg of anthocyanins for 24 weeks were examined, purified anthocyanin improving dyslipidemia, enhancing antioxidant capacity, and preventing insulin resistance(Li et al. Citation2015). In subject with T2DM, blood glucose and antioxidant capacity were improved, and lipid peroxidation and inflammatory responses were alleviated by freeze-dried strawberry intake for six weeks(Moazen et al. Citation2013). In a pomegranate juice study examining subjects with T2DM, after a 12-hour fast, subjects were given 1.5 mL/kg body weight pomegranate juice and then serum glucose levels were measured in 1 h and 3 h after the consumption of the juice. Results showed that subjects’ fasting blood glucose decreased, beta-cell function and insulin resistance improved(Banihani et al. Citation2014). In another clinical study, individuals with T2DM were given 0.47 g bilberry extract for three weeks. The postprandial glycemia decreased while insulin levels did not change(Alnajjar et al. Citation2020).
Mechanism of anthocyanins prevention and treatment of type 2 diabetes
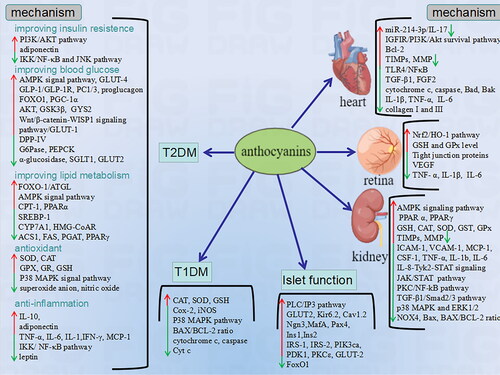
With progression of insulin resistance, lipid metabolism disorders intensify, and blood glucose increases that promote inflammation and oxidative stress ultimately leading the progression of T2DM and the appearance of various complications. These above features of DM are often already present in prediabetes. Anthocyanins improve these features through various mechanisms to prevent prediabetes from progressing to DM. These mechanisms also involve the treatment of T2DM. Thus, we discussed the mechanism of anthocyanins in the prevention and treatment of T2DMtogether ().
Figure 2. The mechanism of health benefits of anthocyanins on diabetes mellitus and pancreatic islets.
NF-κB: nuclear factor kappa-B; cJNK: c-Jun N-terminal kinase; AMPK: AMP-activated protein kinase; GLP-1: Glucagon-like peptide-1; PC1/3: prohormone convertase 1/3; FoxO1: forkhead box O1; PGC-1α: Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; GSK3β: Glycogen Synthase Kinase 3β; GYS2: Glycogen Synthase 2; G6Pase: glucose 6-phosphatase; PEPCK: Phosphoenolpyruvate carboxykinase; ATGL: Adipose triglyceride lipase; CPT-1: Carnitine palmitoyl transferase I; PPAR: peroxisome proliferator-activated receptor; SREBP-1: sterol regulatory element binding protein; CYP7A1: Cytochrome P450 Family 7 Subfamily A Member 1; MMP: Matrix metalloproteinase; TIMP: Tissue matrix metalloproteinase; ICAM-1: leukocyte adhesion factors; VCAM-1: vascular cell adhesion molecule-1; Monocyte chemoattractant protein-1; Ngn3: neurogenin3; IRS: insulin receptor substrate; PIK3ca: phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PDK1: Phosphoinositide-dependent kinase-1;
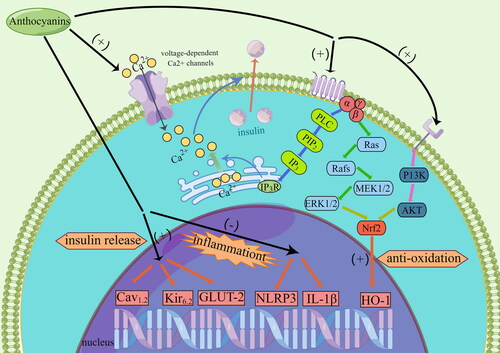
improving insulin resistance:
PI3K/AKT pathway, the insulin signal transduction pathway, accelerates glycogen synthesis and restrain the gluconeogenesis. Proinflammatory cytokines can suppress PI3K/AKT pathway by activation of the inhibitor of kappa B kinase (IKK)/nuclear Factor kappa B(NF-κB) pathway. Anthocyanins can reduce the activation of proinflammatory factors through their anti-inflammatory effects, but also directly activate the P13K/AKT pathway to improve insulin resistance(Yan, Dai, and Zheng Citation2016, Daveri et al. Citation2018). Furthermore, phosphorylation of insulin receptor substrate 1 (IRS-1) serine residues also inhibits insulin signaling causing insulin resistance. The activation of Jun NH2-terminal kinase (JNK) and NF-κB is inhibited by anthocyanins to reduce the phosphorylation of IRS-1 serine residues (Daveri et al. Citation2018, Lee et al. Citation2018). In addition, anthocyanin has been shown to induce adiponectin, which has some insulin-sensitizing properties thus this may be one of the mechanisms for improving insulin resistance(Liu et al. Citation2014, Li et al. Citation2015, Matsukawa et al. Citation2015, Yan, Dai, and Zheng Citation2016, Nemes et al. Citation2019, Yang et al. Citation2020).
improve blood glucose levels:
Currently, anthocyanins improve the hyperglycemic state of T2DM through various mechanisms to decrease the risks of developing T2DM. First, anthocyanins activate AMP-activated protein kinase (AMPK) which is a key molecule in the regulation of bioenergy metabolism in white adipose tissue, skeletal muscle, and the liver(Takikawa et al. Citation2010, Kurimoto et al. Citation2013, Choi et al. Citation2016, Iizuka et al. Citation2018, Jiang et al. Citation2020). In white adipose tissue and skeletal muscle, activation of AMPK induces glucose transporters 4(GLUT4) to enhance glucose uptake and utilization(Sasaki et al. Citation2007, Iizuka et al. Citation2018). In the liver, activation of AMPK reduces glucose production. Second, anthocyanins as participants in glucagon-like peptide-1(GLP-1)/GLP-1 receptor (GLP-1R) related signaling pathway(Tsuda Citation2017, Daveri et al. Citation2018). GLP-1, a significant molecular target for the treatment of T2DM, produced in the gut binds to the GLP-1R on pancreatic β cells, then activates the cAMP/PKA pathway to promote release of insulin and growth of β cells (Jones et al. Citation2018).And anthocyanins increase the secretion of GLP-1 by upregulation of prohormone convertase 1/3 (PC1/3)and proglucagon that are involved in the production of GLP-1(Iizuka et al. Citation2018, Tian et al. Citation2020). Third, anthocyanins lower blood glucose by regulating glycogen metabolism. The activities of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase(G6Pase) inhibited by anthocyanins through upregulating mRNA expression levels of peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α (PGC-1α) and forkhead box protein O1 (FoxO1), thereby reducing glycogen breakdown(Alvarado, Schoenlau, et al. Citation2016, Jiang et al. Citation2020). Furthermore, phosphorylation of AKT and glycogen synthase kinase-3β (GSK3β) are activated by anthocyanins, leading to the upregulation of glycogen synthase 2 (GYS2)(Yan, Dai, and Zheng Citation2016, Herrera-Balandrano et al. Citation2021). And anthocyanins inhibitα-amylase activity that is present in the lumen of the small intestine to improve postprandial hyperglycemia(Yamane et al. Citation2016, Chen et al. Citation2018, Alnajjar et al. Citation2020).Through the glucose transporters 2 (GLUT2) and sodium/glucoseco transporter - 1 (SGLT1), glucose can be absorbed from the small intestine into the blood vessels, thus causing postprandial hyperglycemia(Williamson Citation2013). Some studies have confirmed that anthocyanins can inhibit glucose transporters (SGLT1 and GLUT2) to reduce the absorption of glucose in the intestine, improving postprandial blood glucose(Hidalgo et al. Citation2014, Jiang et al. Citation2020, Herrera-Balandrano et al. Citation2021). In addition to GLUT-4 and GLUT-2, study have shown that anthocyanins may up-regulate the expression of GLUT-1 by activating the Wnt/β-catenin-WISP1 signaling pathway, thereby reducing blood glucose levels(Ye et al. Citation2022).
improving lipid metabolism:
Several studies have reported multiple mechanisms of anthocyanins in improving lipid metabolism. First, many enzymes involved in fatty acid and triacylglycerol synthesis and metabolism, such as Acyl-CoA synthase1(ACS1), fatty acid synthase (FAS), and glycerol-3-phosphate acyltransferase (GPAT), can be inhibited by anthocyanins(Wu, Jiang, et al. Citation2016, Song, Shen, Wang, et al. Citation2021, Song, Shen, Wang, et al. Citation2021). And sterol regulatory element binding protein (SREBP-1) is regulated by insulin and involved in lipase expression. Anthocyanins reduced the expression of SREBP-1 to decrease the fatty acid synthesis(Park et al. Citation2015). Secondly, anthocyanins activated the gene expression of cholesterol metabolism-related enzymes such as cholesterol 7α-hydroxylase (CYP7A1) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoAR). Promoting cholesterol breakdown over cholesterol synthesis, thereby improving the blood lipid parameters of DM(Watanabe and Ayugase Citation2010). Some studies also showed anthocyanins play anti-obesity effects by enhancing antioxidant capacity and improving inflammation levels(Farrell et al. Citation2015, Wu et al. Citation2018, Nemes et al. Citation2019). Third, anthocyanins may improve adipocyte metabolism by regulating transcription factor FoxO1-mediated transcription of adipose triglyceride lipase (ATGL) (Guo, Guo, Guo, et al. Citation2012). Fourth, anthocyanins were shown in other studies to reduce hepatic triglyceride accumulation and improve lipid metabolism by activating AMPK signaling pathways that are key regulators of hepatic lipid metabolism(Hwang et al. Citation2011, Saito et al. Citation2016). Fifth, anthocyanins also inhibited the expressions of adipogenic genes such as PPARγ and enhanced the expression of carnitine palmitoyl transferase (CPT-1) and PPARα, which are associated with fat oxidation(Seymour et al. Citation2011, Farrell et al. Citation2015, Noratto et al. Citation2018, Lim et al. Citation2019, Park et al. Citation2019, Kim et al. Citation2020, Lee et al. Citation2021).
improving oxidative stress:
Cell damage-induced by oxidative stress plays an important role in progression of T2DM, so the antioxidant capacity of anthocyanins is also one of the reasons for the anti-T2DM effect. Anthocyanins scavenge excess free radicals and reduce damage caused by oxidative stress by activating endogenous antioxidant defense systems(Li et al. Citation2015). Studies have consistently shown that anthocyanins improve the activity of enzymatic antioxidants in the body, such as superoxide dismutase (SOD) and catalase (CAT), enhance their ability to protect cells from oxidative damage by catalyzing the conversion of free radicals into hydrogen peroxide (Chen et al. Citation2018, Nemes et al. Citation2019, Ye et al. Citation2021). In addition, anthocyanins also enhance Glutathione peroxidase (GPx) and glutathione reductase (GR) activity, and accelerate the synthesis of glutathione (GSH), ultimately improving plasma antioxidant activity in T2DM populations(Chen et al. Citation2018, Qin et al. Citation2018). Oxidative stress has been shown to activate P38 MAPK signaling pathway, which play a crucial role in oxidative stress response. And the activation of P38 MAPK can be inhibited by anthocyanins(Liu et al. Citation2020). Andeven research shown that anthocyanins indirectly exert antioxidant effects by regulating the production of the superoxide anion and nitric oxide through their metabolites(Daveri et al. Citation2018).
improving inflammation:
Long-term Inflammatory response may contribute to insulin resistance eventually leading to T2DM, and promote the progression of DM complications(Lontchi-Yimagou et al. Citation2013). Some inflammatory cytokines TNF-α, IL-6, IL-1 play a key role in the inflammatory response, while anthocyanins can inhibit the expression of these inflammatory cytokines, thus playing an anti-inflammatory role(Qin and Anderson Citation2012, Farrell et al. Citation2015, Hsu et al. Citation2016, Wu, Jiang, et al. Citation2016, Qin et al. Citation2018, Nemes et al. Citation2019). Monocyte chemoattractant protein 1 (MCP-1) is a chemokine that regulates the migration and infiltration of leukocytes and is involved in the pathogenesis of DM(Mussa et al. Citation2021). Many studies have proved that anthocyanins can reduce the expression of MCP-1(Farrell et al. Citation2015, Nemes et al. Citation2019). And anthocyanins also inhibit expression of these inflammatory cytokines gene by suppressing IKK/NF-κB pathway(Sarikaphuti et al. Citation2013, Daveri et al. Citation2018, Huang et al. Citation2018, Wu et al. Citation2018). At the same time, the secretion of adiponectin increased by anthocyanins, thereby reducing TNF-α and IFN-γ expression, stimulating the production of anti-inflammatory IL-10, and converting the phenotype of macrophages from proinflammatory type to the anti-inflammatory type(Oyama et al. Citation2016, Nemes et al. Citation2019). In addition, leptin produced by adipocytes can regulate immune response, including activation of immune cells and induction of pro-inflammatory mediators(Maya-Monteiro and Bozza Citation2008). And anthocyanins have been shown in multiple studies to inhibit leptin production(Sarikaphuti et al. Citation2013, Yan, Dai, and Zheng Citation2016, Nemes et al. Citation2019, Nikbakht et al. Citation2021).
Anthocyanins and complication of diabetes mellitus
DM and its macrovascular and microvascular complications cause enormous psychological and physical distress to patients and a huge burden on social care systems. Several studies have demonstrated positive effects of anthocyanins on DM complications ().
Table 3. The treatment of anthocyanins on diabetic complications in animal trial.
Heart complications
Cardiovascular complications are the leading cause of death and disability in people with DM(Lin et al. Citation2014). The main risk factor for cardiovascular disease in T2DM is Dyslipidemia, which is characterized by low HDL-cholesterol concentrations, and high LDL-cholesterol concentrations and TGs concentrations(Li et al. Citation2015). By causing chronic inflammation of the heart, hypertrophy, apoptosis and fibrosis, DM eventually leads to myocardial remodeling, deteriorated cardiac function, and even most debilitating prognosis––heart failure(Chen et al. Citation2016).
Platelets play an important role in the development of atherosclerosis and consequently CVD in DM, and anthocyanins have potential antiplatelet ability that subsequently can prevent atherosclerosis and CVD(Gaiz et al. Citation2018). Bilberries and blueberries, anthocyanin-rich fruits, have the potential to curb cardio-metabolic perturbations(Crespo and Visioli Citation2017). In animal research on anthocyanins from purple rice, the extent of myocardial injury and cardiac function loss in mice with DM caused by STZ-induced decreased after 4 weeks of diet anthocyanins, and myocardial hypertrophy and fibrosis were significantly reduced(Chen et al. Citation2016). STZ-induced rats that consumed anthocyanins had enhanced cardiac function by improving inflammation and fibrosis in the heart tissue (Yue et al. Citation2020). In another study, STZ-induced rats enhanced cardiomyocyte survival and restores cardiac function after receiving 4 weeks of anthocyanin treatment(Huang et al. Citation2017). In addition, protocatechuic acid, a main metabolite of anthocyanin, was confirmed to have potential protective effects on the hearts of STZ-induced T1DM rats, mainly including improving cardiac function, preventing myocardial mitochondrial dysfunction, and increasing B-cell lymphoma-2 expression (Semaming et al. Citation2014).
Mechanism
High glucose induced inflammation and myocardial fibrosis are two major pathogenic factors of diabetic cardiac complications.Interleukin-17(IL-17), as a pro-inflammatory cytokine, is known to play a critical role in cardiac fibrosis(Valente et al. Citation2012). Anthocyanins can reduce the expression of IL-17 by regulating miR-214-3p, which is a short non-coding RNAs that controls the process of inflammation, thus protecting cardiac function(Yue et al. Citation2020). Metalloproteinase (MMPs) can promote the transformation of fibroblasts into myofibroblasts leading to myocardial remodeling, and this effect can be inhibited by their tissue inhibitors (TIMPs) (Hansson et al. Citation2009). Anthocyanins can regulate homeostatic equilibrium between MMP and TIMP-1 and inhibit transforming growth factor β(TGF-β) and fibroblast Growth Factor 2 (FGF2) that are associated with cardiac fibrosis(Chen et al. Citation2016). Chen, Y. F et al also found that anthocyanins reduced toll-like receptors-4(TLR4)/NF-κB activation and improved myocardial inflammation and function. Hyperglycemia-induced cardiomyocyte necrosis and apoptosis is also one of the pathogeneses of diabetic cardiac complications. IGFI-R/PI3K/AKT survival signaling, and Bcl-2 family associated anti-apoptotic mechanisms are enhanced by anthocyanins, while caspase family and Bad and Bak proteins related to apoptosis mechanisms are suppressed(Huang et al. Citation2017).
Diabetic nephropathy
DN is one of the main complications of DM, and it is also the main cause of end-stage renal disease in the world(Qi et al. Citation2017). Hyperglycemia and inflammation pathways play a significant role in the progression of DN, which is characterized by accumulation of extracellular matrix proteins and irreversible decline in renal function(Wada and Makino Citation2013). Purple corn extract inhibited glomerular monocyte activation, macrophage infiltration, mesangial hyperplasia, and collagen fiber accumulation and ameliorated severe albuminuria in db/db mice(Kang et al. Citation2012, Li, Kang et al. Citation2012). In a study where STZ-induced C57BL/6J mice were fed a high fat diet and varied concentrations of myrciaria cauliflora extracts rich in anthocyanins, after treatment mice had improved renal injury and decreased accumulation of saccharide and formation of collagen IV (Hsu et al. Citation2016, Wu, Hung, et al. Citation2016). Similar result was observed in rats with DN(Zheng et al. Citation2020). After db/db mice were treated with C3G, renal function and histology were improved, and renal hypertrophy was reduced. Moreover, C3G exhibits downregulation of glucose level, hypolipidemic effect, antioxidant effect, and anti-inflammatory effect in db/db mice, which may play a protective role against DN (Qin et al. Citation2018, Wei et al. Citation2018). Kidney damage and the oxidative DNA damage in Zucker diabetic fatty rat were alleviated after supplementation with red orange and lemon extract through gavage for 24 weeks(Damiano et al. Citation2020). DIAVIT, a blueberry and sea buckthorn extract, inhibited the development of albuminuria and the enhancement of glomerular water permeability and prevented fibrotic glomeruli, endothelial insult, and glomerular ultra-structural changes(Stevens et al. Citation2019). Anthocyanins also improved intra-renal lipid concentrations and reduced renal apoptosis and oxidative stress in db/db mice with improvement of glomerular inflammation(Koh et al. Citation2015). However, in another animal experiment, kidney inflammation did not significantly improve(Damiano et al. Citation2019).
Protocatechuic acid, an anthocyanin metabolite, was shown in an in vitro study to ameliorates extracellular matrix accumulation to prevent DN(Ma, Chen et al. Citation2018). In many vitro studies, purple corn extract was shown that suppressed renal mesangial fibrosis and inflammation induced by high glucose and prevented glomerular angiogenesis (Li, Kang et al. Citation2012, Li, Lim, et al. Citation2012, Kang et al. Citation2013).
Mechanism
Renal function can be improved by anthocyanins via deceasing renal inflammation and enhancing the ability of antioxidative(Koh et al. Citation2015, Hsu et al. Citation2016, Qin et al. Citation2018, Zheng et al. Citation2020). NF-κB plays a pivotal role in the process of cellular inflammation and immune response that triggers renal fibrosis(Manucha Citation2014). After oxidative stress was ameliorated by anthocyanins, a decrease in the expression of the PKC enzymes/NF-κB that mediated DN(Hsu et al. Citation2016). The Ras pathway is extensively activated in renal cells by high glucose, promoting cell proliferation and epithelial-mesenchymal transition leading to renal fibrosis(Hirose et al. Citation2010). Anthocyanins can reduce Ras-related protein expression and inhibit Ras/PI3K/Akt signaling pathway to ameliorate renal mesangial fibrosis(Wu, Hung, et al. Citation2016). The IL-8/Tyk2/STAT signaling pathway plays a major role in the progression of DN by promoting renal mesangial fibrosis and inflammatory cell infiltration, while this pathway can be blocked by anthocyanins(Kang et al. Citation2012, Li, Kang et al. Citation2012). In addition, anthocyanins can also inhibit the TGF-β1/Smad2/3 pathway that is involved in ECM synthesis and accumulation to improve renal fibrosis(Zheng et al. Citation2020).Apoptosis of kidney cells is also one of the main pathogeneses of DN(Sanchez-Niño et al. Citation2010). And this cell apoptosis can be attenuated by anthocyanins by inhibiting the expression of apoptosis-related proteins, such as caspase-3, modulating the Bax/Bcl-2 ratio, and reducing phosphorylation of p38 MAPK and ERK1/2(Wei et al. Citation2018, Damiano et al. Citation2020).
Diabetic retinopathy
DR, a serious complication of DM, is considered the leading cause of vision loss and blindness worldwide(Singh et al. Citation2019). DR can be divided into non-proliferative retinopathy (NPDR) and proliferative DR (PDR) based on different phases. The characterization of DR is dysfunction of retinal blood vessels and breakdown of retinal blood barrier, which can be caused by oxidative stress and chronic inflammation induced by altered metabolites and hormones in DM(Zhang, Liu, et al. Citation2011, Preedy Citation2014).
In a blueberry study observing STZ-induced rat, after 12 weeks of taking either 20, 40, or 80 mg/kg, blood–retinal barrier breakage improved in rat retinas of all groups taking blueberry anthocyanins(Song, Huang, and Yu Citation2016). At that time STZ-induced rat received gastric gavage of bilberries extract had lower the fluorescein leakage in the fluorescein-dextran angiography and the level of retinal vascular endothelial growth factor (VEGF) that a remark of DR than control group without bilberries extract(Kim, Kim et al. Citation2015). Similar results were observed in another study that retinal vascular leakage was attenuated after STZ-induced diabetic mice were administered with C3G. In addition, C3G can inhibit retinal glial cell proliferation and angiogenesis(Zhao et al. Citation2021).
Mechanism
Anthocyanins may have protective effects on retina by their antioxidant and anti-inflammatory properties. For example, anthocyanins may regulate Nrf2/heme oxygenase-1 (HO-1) signaling pathway to improve antioxidant capacity and inflammation levels in retinal cells(Song, Huang, and Yu Citation2016). Furthermore, anthocyanins inhibit the mRNA and protein expression of VEGF that is related to blood–retinal barrier breakage to prevent DR(Kim, Kim et al. Citation2015). Microglia, which are monocytes resident in the retina, have been shown to play an important role in diabetic retinopathy(Altmann and Schmidt Citation2018). And anthocyanins regulate activation of microglia to delay the process of DR. In addition, anthocyanins improve retinol vascular permeability by increasing the expression of tight junction proteins, such as occludin-1, claudin-1, and ZO-1(Kim, Kim et al. Citation2015, Zhao et al. Citation2021).
Type 1 diabetes mellitus
Animal trial
In this section, we analyzed the benefits of anthocyanins for T1DM (). Overnight-fasted mice induced by single streptozotocin (STZ), which inhibits insulin secretion, can be used as an animal model of T1DM to explore the mechanism of action. In a study where STZ-induced diabetic mice were fed by normal diet or normal diet plus 10 mg/kg aronia berry (contains a large amount of natural antioxidant) extract or normal diet plus 100 mg/kg aronia berry extract, finally mice receiving normal plus 100 mg/kg aronia berry extract had better lipid and blood glucose metabolic markers and better insulin levels(Jeon et al. Citation2018). Anthocyanins from other four plants have also been shown to reduce blood glucose and blood lipids in STZ-Induced animal research(Strugała, Dzydzan et al. Citation2019, Vilhena et al. Citation2020). Meanwhile, the fasting glucose of delphinol-treated STZ-induced rats was significantly improved(Hidalgo et al. Citation2014). The extracts of red and yellow fruits of cornelian cherries, containing a significant number of anthocyanins, were used to feed rats with STZ-induced T1DM. After 14 days of feeding, rats dosed with the extracts had lower blood glucose concentrations and amounts of glycated hemoglobin, greater erythrocyte resistance to acid hemolysis, and higher reduced glutathione and mean red blood cell levels than control groups(Dzydzan et al. Citation2019). In another studies, rats with STZ-induced diabetes were given red cabbage extract, and after 4-week rats also received similar antidiabetic effects, and islet morphology and beta cell numbers also were restored (Buko et al. Citation2018). Yue et al proved that anthocyanins not only reduced blood glucose in T1DM mice, but also delayed progress in cardiovascular complications of DM(Yue et al. Citation2020). Nizamutdinova et al observed that anthocyanins from black soybean seed coats improved blood glucose and lipids and insulin resistance, reduced islet cell apoptosis, enhanced antioxidant capacity, and even improved cardiac function in STZ-induced rats(Nizamutdinova et al. Citation2009).
Mechanism
Autoimmunity, a major pathogenesis of T1DM, can not only directly lead to islet cell damage and destruction, but also release inflammatory cytokines to cause islet inflammation, which gradually leads to the progression of T1DM(Bending, Zaccone, and Cooke Citation2012). Furthermore, oxidative stress induced by hyperglycemia could cause islet β-cell apoptosis and exacerbate the inflammatory response via promoting the expression of inflammatory cytokines and chemokines(Novoselova et al. Citation2021). Cyclooxygenase-2 (COX-2) is the key to triggering inflammation, while inducible iNOS synthesizes large amounts of NO that lead to aggressive oxidative stress. Anthocyanins block mRNA expression level of COX-2 and iNOS thereby improving inflammation and oxidative stress(Jeon et al. Citation2018). In addition, improving the expression of some antioxidant enzymes, such as CAT, SOD, and GSH, is also anti-T1DM mechanism of anthocyanins(Soon and Tan Citation2002, Buko et al. Citation2018, Strugała, Dzydzan et al. Citation2019). The P38 MAPK pathway that is involved in apoptosis can be activated by oxidative stress. Some proteins including Bcl-2 family, Cyt-c and caspase-3 are negatively regulated by upstream protein MAPK, thus lead to apoptosis of cells(Fu et al. Citation2006). Anthocyanins can inhibit the activation of P38 MAPK pathways and regulate Bax/Bcl-2 ratios and caspase-3 activity to improve islet cell apoptosis and oxidative stress to maintain islet function(Nizamutdinova et al. Citation2009, Lee, Kim et al. Citation2015). In addition, anthocyanins can reduce the production of ROS by controlling blood glucose(Dzydzan et al. Citation2019).
Anthocyanins and islet function and transplantation
Pancreatic islets, which are composed of a group of endocrine cells, perform many functions by releasing hormones. The most important function is regulated glucose metabolism, with hyperglycemia-stimulating insulin release by β-cells and decrease in glycemia-triggering glucagon secretion by α-cells(Thorens Citation2014). Part of anthocyanins have been shown to be capable of promoting insulin secretion at 4 and 10 mM glucose concentrations in pancreatic β-cells(Jayaprakasam et al. Citation2005). Similar results were reported on isolated mice pancreatic islets in the presence of 5.5 mM and 16.5 mM glucose concentration(Doshi et al. Citation2015). Anthocyanins from purple corn have also been shown to promote insulin secretion in iNS-1E cells(Luna-Vital and Gonzalez de Mejia Citation2018). In an in vitro study, cyanidin has proven to stimulate islet β-cells to release insulin through by Ca2+ influx via voltage-dependent Ca2+ channels and showed dose response relationship. At the same time, cyanidin increased the expression of genes, such as GLUT2, Kir6.2 and Cav1.2, which are associated with insulin release(Suantawee et al. Citation2017). In addition to voltage-dependent Ca 2+ channels, anthocyanin also promoted insulin release through activating the PLC/IP3 pathway(Kongthitilerd et al. Citation2022). Blueberry-leaf extract reduced compensatory increase in the pancreatic islet size and insulin content induced by hyperglycemia. At that time the expression of β-cell proliferation-related genes increased and the expression of β-cell apoptosis-related genes inhibited after high fat diet-fed mice were given blueberry-leaf extract (Li et al. Citation2020). Anthocyanins prevented pancreatic cell apoptosis in STZ-induced diabetic rats by regulating apoptosis-related proteins(Nizamutdinova et al. Citation2009). In STZ-induced diabetic rats, anthocyanins can also protect the islet morphology and degeneration, and activate insulin receptor phosphorylation to restore the number of β-cells(Jayaprakasam et al. Citation2006, Nizamutdinova et al. Citation2009, Nasri et al. Citation2011, Sarikaphuti et al. Citation2013, Buko et al. Citation2018). In addition, after supplementation with anthocyanins in diabetic animal models, the antioxidant capacity in pancreas islets was improved, thus reducing islet cell damage, and improving islet function(Ji et al. Citation2015, Ye et al. Citation2021).
Previously, our group demonstrated that anthocyanins from Chinese bayberry have a positive effect on improving the antioxidant capacity of islet cells after islet transplantation(Zhang, Kang, et al. Citation2011, Zhang et al. Citation2013, Cai et al. Citation2015, Li, Yang, et al. Citation2017, Croden et al. Citation2021). In an in vitro study, pretreatment of INS-1 cells with anthocyanins reduced cell death caused by H2O2 compared to the control group. In subsequent animal experiments, anthocyanins were shown to effectively reduce graft apoptosis in the β-cell transplantation STZ-induced mice by up-regulating HO-1 expression through via activation of PI3K/Akt and ERK1/2(Zhang, Kang, et al. Citation2011). Oxidative stress-mediated autophagic cell death in INS-1 cells and grafts was reduced after pretreated with anthocyanins(Zhang et al. Citation2013). C3G, a major anthocyanin component of Chinese bayberry, was used to treat islets from B6 mice before transplantation. After transplantation, islets treated with C3G showed stronger viability and function than untreated islets(Cai et al. Citation2015). In an animal experiment, transplantation of C3G-treated porcine islets into STZ-induced mice further demonstrated our previous study that anthocyanins reduced damage caused by oxidative stress and enhanced islet function after islet transplantation(Li, Yang, et al. Citation2017). In vitro, C3G was shown to effectively reduce the expression of inflammatory markers IL-1β and OD-like receptor thermal protein domain associated protein 3 (NLRP3) and increase the expression of LC3 autophagic marker and HO-1 in human islet after transplantation, and thus may play a protective role in islet cell(Croden et al. Citation2021). The mechanism by which anthocyanins improve islet transplantation and islet function was shown in .
Future perspective
A growing body of scientific evidence carried out on animals demonstrates that anthocyanins play a role in prevention and treatment of DM. And different mechanisms have been raised to explain anti-diabetic effect of anthocyanins, but most of the mechanism lacks further clinical studies to prove that, even if there is, the results are not entirely meaningful. In addition, anthocyanin intake has been considered to have a dose-response relationship with risk of DM in many systematic review studies. However, the intake quantity of anthocyanins treatment in these studies varied greatly, with some subjects received 320 mg/d or greater, and other under 100 mg/d, and these results may not be applicable to other ethnic groups. Thus, more large-scale human studies are necessary to conclusive evidence of the optimal daily intake of anthocyanin that minimizes the risk of DM.
All the time, the bioavailability of anthocyanins is considered to be extremely low, which contradicts the health benefits of anthocyanins. However, the biological metabolic rate of anthocyanins is severely underestimated, a recovery of 12.4% in a human bioavailability labeled by the cyanidin-3-glucoside(Czank et al. Citation2013). This underestimation is largely due to the facts that anthocyanins are transformed to various molecular in presystemic metabolism before they reach the plasma and the knowledge about the mechanisms of anthocyanin absorption, catabolism and metabolism is very limited(Lila et al. Citation2016). Currently, although different studies have been proposed to better understand of the anthocyanin bioavailability, the metabolized conjugates of these full sets of anthocyanins have not yet been determined. Thus, instruments with greater sensitivity and resolution needs to be designed to precise detection of anthocyanins and more in vivo and in vitro studies are needed to further explore the mechanism about anthocyanin catabolism in body.
Controlled release is an advanced approach to improve the biological activity of anthocyanins, controlling the slow release of anthocyanins at the target site leading concentration of anthocyanins reaches therapeutic level within a period(He, Ge, et al. Citation2017). Lipid-based, polysaccharide-based and protein-based complexes, nanoencapsulation and exosome have been designed to not only promote anthocyanin absorption and release at targeted sites, but also increase anthocyanin efficacy(Shen et al. Citation2022). However, few studies have focused on the controlled release of anthocyanins in the pancreas, which may enhance the ability of anthocyanins for the prevention and treatment of DM. And a growing body of research has demonstrated that direct delivery of antidiabetic drugs to the islet environment via nanocarriers and exosomes is a promising treatment option for T1DM and T2DM(Zeynaloo et al. Citation2022). Thus, it is very meaningful to carry out research on the controlled release of anthocyanins in the pancreas and these new approaches all require further studies to assess safety and efficacy.
The stability of anthocyanins is also the main reason for limiting the application of these compounds. The chemical structure of anthocyanins has a great influence on its stability. Unsaturated double bonds and oxidized groups will reduce its stability, while the methylation, glycosylation and acylation of glycosyl ligands will stabilize it. In recent years, great progress has been made in methods to improve the stability of anthocyanins, such as glycosyl acylation, whey protein-based, nanoemulsion and nanoliposome-based, etc.(Zhao et al. Citation2017, Chen and Stephen Inbaraj Citation2019, Ren, Jiménez-Flores, and Giusti Citation2021). But there are still many problems that need more research to further explore, say, how to solve undesirable exogenous materials be introduced in nanocarrier systems. Furthermore, the more precise and specific approaches need to be developed and large-scale medical application needs more clinical data.
With increasing drug dose, most of anti-diabetic agents caused various adverse reactions, while anthocyanins are of great nutritional interest with no adverse effects were reported. Combining anthocyanins with anti-diabetic agents not only effectively reduce the occurrence of adverse reactions, but also enhance the efficacy. Animal research indicate that synergistic effects of combined anthocyanins and metformin treatment on the blood glucose and insulin resistance(Zou et al. Citation2021, Tian et al. Citation2022). In addition, combined administration of the anthocyanins with fenofibrate enhanced ability of fenofibrate to improve lipid metabolism in patient with T2DM(Kusunoki et al. Citation2015). However, studies on combination therapy of antidiabetic drugs with anthocyanins are still limited. Further research in this direction may lead to anthocyanins as a supplementary treatment of anti-diabetic drugs for clinical application soon.
Few studies reported the benefits of anthocyanins in T1DM, as well as studies on anthocyanins for islet transplantation. Islet transplantation, as an effective treatment of T1DM, is often limited by the effects of oxidative stress produced in isolation and transplantation procedures. At present, anthocyanins were shown that may play a protective in islet after transplantation in vitro and animal studies by regulate the expression of HO-1, inflammatory markers IL-1β and NLRP3, and LC3 autophagic marker. However, these mechanisms need to be demonstrated in further in vivo studies, and more research needs to explore the protective mechanism of anthocyanins on islet transplantation.
Conclusion
The anthocyanins found in some natural plants produce positive effects that have been reported in medicinal field as potential supplementary treatment for DM. Here, this review has comprehensively assessed the healthy effect of anthocyanins on DM as well as its complications. Numerous studies confirm that anthocyanins have beneficial effects on glucose metabolism, lipid metabolism, insulin resistance, antioxidant, and anti-inflammatory through various mechanisms, thus plays a role in the prevention and treatment of DM. In addition, anthocyanins can also reduce organ damage caused by high glucose and lipid deposition, improve organ function, and prevent progression of the complication. At the same time, improvement in islet function and reduction in islet injury after transplantation associate with anthocyanin is demonstrated by animal studies. It can be anticipated that with the development of study in improving the stability of anthocyanins, more research on the effect of anthocyanins on DM will be carried out and the optimal intake of anthocyanin will be determined.
| Abbreviation | ||
| DM | = | Diabetes mellitus |
| DR | = | diabetic retinopathy |
| DN | = | diabetic nephropathy |
| C3G | = | cyanidin-3-glucoside |
| NF-κB | = | nuclear Factor kappa B |
| AMPK | = | AMP-activated protein kinase |
| PPAR | = | peroxisome proliferator-activated receptor |
| ROS | = | reactive oxygen species |
| TLR4 | = | Toll-like receptors 4 |
| TNF-α | = | tumor necrosis factor |
| IL-6 | = | Interleukin 6 |
| GLP-1 | = | Glucagon-like peptide-1 |
| FAS | = | fatty acid synthase |
| TLR4 | = | Toll-like receptors 4 |
| MMP | = | Matrix metalloproteinase |
| TIMP | = | Tissue matrix metalloproteinase |
| IGFIR | = | insulin-like growth factor I receptor |
Supplemental Material
Download MS Word (64.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors. were created by Figdraw (www.figdraw.com).
Additional information
Funding
References
- Alappat, B., and J. Alappat. 2020. Anthocyanin pigments: Beyond aesthetics. Molecules 25 (23):5500. doi: 10.3390/molecules25235500.
- Al-Ishaq, R. K., M. Abotaleb, P. Kubatka, K. Kajo, and D. Büsselberg. 2019. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 9 (9):430. doi: 10.3390/biom9090430.
- Alnajjar, M., S. Kumar Barik, C. Bestwick, F. Campbell, M. Cruickshank, F. Farquharson, G. Holtrop, G. Horgan, P. Louis, K.-M. Moar, et al. 2020. Anthocyanin-enriched bilberry extract attenuates glycaemic response in overweight volunteers without changes in insulin. Journal of Functional Foods 64:103597. doi: 10.1016/j.jff.2019.103597.
- Altmann, C., and M. H. H. Schmidt. 2018. The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. International Journal of Molecular Sciences 19 (1). doi: 10.3390/ijms19010110.
- Alvarado, J. L., A. Leschot, Á. Olivera-Nappa, A. M. Salgado, H. Rioseco, C. Lyon, and P. Vigil. 2016. Delphinidin-rich maqui berry extract (Delphinol®) lowers fasting and postprandial glycemia and insulinemia in prediabetic individuals during oral glucose tolerance tests. BioMed Research International 2016:9070537.
- Alvarado, J., F. Schoenlau, A. Leschot, A. M. Salgad, and P. Vigil Portales. 2016. Delphinol® standardized maqui berry extract significantly lowers blood glucose and improves blood lipid profile in prediabetic individuals in three-month clinical trial. Panminerva Medica 58 (3 Suppl 1):1–6.
- Andersen, Ø. M., M. Jordheim, R. Byamukama, A. Mbabazi, G. Ogweng, I. Skaar, and B. Kiremire. 2010. Anthocyanins with unusual furanose sugar (apiose) from leaves of Synadenium grantii (Euphorbiaceae). Phytochemistry 71 (13):1558–63. doi: 10.1016/j.phytochem.2010.05.025.
- Ashande, C. M., A. Masunda, K. N. Ngbolua, J. T. Kilembe, A. Matondo, I. Liyongo Clément, G. Z. Benjamin, L. M. Emmanuel, D. S. T. Tshibangu, D. D. Tshilanda, et al. 2022. Glucose oxidase as a model enzyme for antidiabetic activity evaluation of medicinal plants: In vitro and in silico evidence.
- Azzini, E., E. Venneria, D. Ciarapica, M. S. Foddai, F. Intorre, M. Zaccaria, F. Maiani, L. Palomba, L. Barnaba, C. Tubili, et al. 2017. Effect of red orange juice consumption on body composition and nutritional status in overweight/obese female: A pilot study. Oxidative Medicine and Cellular Longevity 2017:1–9. doi: 10.1155/2017/1672567.
- Banihani, S. A., S. M. Makahleh, Z. El-Akawi, R. A. Al-Fashtaki, O. F. Khabour, M. Y. Gharibeh, N. A. Saadah, F. H. Al-Hashimi, and N. J. Al-Khasieb. 2014. Fresh pomegranate juice ameliorates insulin resistance, enhances β-cell function, and decreases fasting serum glucose in type 2 diabetic patients. Nutrition Research 34 (10):862–7. doi: 10.1016/j.nutres.2014.08.003.
- Bending, D., P. Zaccone, and A. Cooke. 2012. Inflammation and type one diabetes. International Immunology 24 (6):339–46. doi: 10.1093/intimm/dxs049.
- Beulens, J., F. Rutters, L. Rydén, O. Schnell, L. Mellbin, H. E. Hart, and R. C. Vos. 2019. Risk and management of pre-diabetes. European Journal of Preventive Cardiology 26 (2_suppl):47–54. doi: 10.1177/2047487319880041.
- Boto-Ordóñez, M., M. Urpi-Sarda, M. I. Queipo-Ortuño, S. Tulipani, F. J. Tinahones, and C. Andres-Lacueva. 2014. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food & Function 5 (8):1932–8. doi: 10.1039/C4FO00029C.
- Buchweitz, M., J. Brauch, R. Carle, and D. R. Kammerer. 2013. Application of ferric anthocyanin chelates as natural blue food colorants in polysaccharide and gelatin based gels. Food Research International 51 (1):274–82. doi: 10.1016/j.foodres.2012.11.030.
- Buko, V., I. Zavodnik, O. Kanuka, E. Belonovskaya, E. Naruta, O. Lukivskaya, S. Kirko, G. Budryn, D. Żyżelewicz, J. Oracz, et al. 2018. Antidiabetic effects and erythrocyte stabilization by red cabbage extract in streptozotocin-treated rats. Food & Function 9 (3):1850–63. doi: 10.1039/c7fo01823a.
- Cai, H., B. Yang, Z. Xu, B. Zhang, B. Xu, X. Li, P. Wu, K. Chen, R. V. Rajotte, Y. Wu, et al. 2015. Cyanidin-3-O-glucoside enhanced the function of syngeneic mouse islets transplanted under the kidney capsule or into the portal vein. Transplantation 99 (3):508–14. doi: 10.1097/TP.0000000000000628.
- Cao, H., J. Ou, L. Chen, Y. Zhang, T. Szkudelski, D. Delmas, M. Daglia, and J. Xiao. 2019. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Critical Reviews in Food Science and Nutrition 59 (20):3371–9. doi: 10.1080/10408398.2018.1492900.
- Chan, S. W., T. T. W. Chu, S. W. Choi, I. F. F. Benzie, and B. Tomlinson. 2021. Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in Chinese patients with type 2 diabetes. Phytotherapy Research: PTR 35 (6):3236–45. doi: 10.1002/ptr.7038.
- Chellappan, D. K., W. S. Yap, N. A. Bt Ahmad Suhaimi, G. Gupta, and K. Dua. 2018. Current therapies and targets for type 2 diabetes mellitus. Panminerva Medica 60 (3):117–31. doi: 10.23736/S0031-0808.18.03455-9.
- Chen, B. H., and B. Stephen Inbaraj. 2019. Nanoemulsion and Nanoliposome Based Strategies for Improving Anthocyanin Stability and Bioavailability. Nutrients 11 (5):1052. doi: 10.3390/nu11051052.
- Chen, Y. F., M. A. Shibu, M. J. Fan, M. C. Chen, V. P. Viswanadha, Y. L. Lin, C. H. Lai, K. H. Lin, T. J. Ho, W. W. Kuo, et al. 2016. Purple rice anthocyanin extract protects cardiac function in STZ-induced diabetes rat hearts by inhibiting cardiac hypertrophy and fibrosis. The Journal of Nutritional Biochemistry 31:98–105. doi: 10.1016/j.jnutbio.2015.12.020.
- Chen, Z., C. Wang, Y. Pan, X. Gao, and H. Chen. 2018. Hypoglycemic and hypolipidemic effects of anthocyanins extract from black soybean seed coat in high fat diet and streptozotocin-induced diabetic mice. Food & Function 9 (1):426–39. doi: 10.1039/C7FO00983F.
- Chien, C.-Y., and B.-D. Hsu. 2013. Optimization of the dye-sensitized solar cell with anthocyanin as photosensitizer. Solar Energy 98:203–11. doi: 10.1016/j.solener.2013.09.035.
- Choi, K. H., H. A. Lee, M. H. Park, and J. S. Han. 2016. Mulberry (Morus alba L.) fruit extract containing anthocyanins improves glycemic control and insulin sensitivity via activation of AMP-activated protein kinase in diabetic C57BL/Ksj-db/db mice. Journal of Medicinal Food 19 (8):737–45. doi: 10.1089/jmf.2016.3665.
- Cole, J. B., and J. C. Florez. 2020. Genetics of diabetes mellitus and diabetes complications. Nature Reviews. Nephrology 16 (7):377–90. doi: 10.1038/s41581-020-0278-5.
- Crespo, M. C., and F. Visioli. 2017. A brief review of blue- and bilberries’ potential to curb cardio-metabolic perturbations: Focus on diabetes. Current Pharmaceutical Design 23 (7):983–8. doi: 10.2174/1381612822666161010120523.
- Croden, J., J. R. Silva, W. Huang, N. Gupta, W. Fu, K. Matovinovic, M. Black, X. Li, K. Chen, Y. Wu, et al. 2021. Cyanidin-3-O-Glucoside improves the viability of human islet cells treated with amylin or Aβ1-42 in vitro. Plos One 16 (10):e0258208. doi: 10.1371/journal.pone.0258208.
- Czank, C., A. Cassidy, Q. Zhang, D. J. Morrison, T. Preston, P. A. Kroon, N. P. Botting, and C. D. Kay. 2013. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. The American Journal of Clinical Nutrition 97 (5):995–1003. doi: 10.3945/ajcn.112.049247.
- Damiano, S., C. Lauritano, C. Longobardi, E. Andretta, A. M. Elagoz, P. Rapisarda, M. D. Iorio, S. Florio, and R. Ciarcia. 2020. Effects of a red orange and lemon extract in obese diabetic zucker rats: Role of nicotinamide adenine dinucleotide phosphate oxidase. Journal of Clinical Medicine 9 (5). doi: 10.3390/jcm9051600.
- Damiano, S., P. Lombari, E. Salvi, M. Papale, A. Giordano, M. Amenta, G. Ballistreri, S. Fabroni, P. Rapisarda, G. Capasso, et al. 2019. A red orange and lemon by-products extract rich in anthocyanins inhibits the progression of diabetic nephropathy. Journal of Cellular Physiology 234 (12):23268–78. doi: 10.1002/jcp.28893.
- Daveri, E., E. Cremonini, A. Mastaloudis, S. N. Hester, S. M. Wood, A. L. Waterhouse, M. Anderson, C. G. Fraga, and P. I. Oteiza. 2018. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biology 18:16–24. doi: 10.1016/j.redox.2018.05.012.
- DeJesus, R. S., C. R. Breitkopf, L. J. Rutten, D. J. Jacobson, P. M. Wilson, and J. S. Sauver. 2017. Incidence rate of prediabetes progression to diabetes: Modeling an optimum target group for intervention. Population Health Management 20 (3):216–23. doi: 10.1089/pop.2016.0067.
- Doshi, P., P. Adsule, K. Banerjee, and D. Oulkar. 2015. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L) byproducts. Journal of Food Science and Technology 52 (1):181–90. doi: 10.1007/s13197-013-0991-1.
- Duarte, L. J., V. C. Chaves, M. Nascimento, E. Calvete, M. Li, E. Ciraolo, A. Ghigo, E. Hirsch, C. M. O. Simões, F. H. Reginatto, et al. 2018. Molecular mechanism of action of Pelargonidin-3-O-glucoside, the main anthocyanin responsible for the anti-inflammatory effect of strawberry fruits. Food Chemistry 247:56–65. doi: 10.1016/j.foodchem.2017.12.015.
- Dzydzan, O., I. Bila, A. Z. Kucharska, I. Brodyak, and N. Sybirna. 2019. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food & Function 10 (10):6459–72. doi: 10.1039/c9fo00515c.
- Eizirik, D. L., L. Pasquali, and M. Cnop. 2020. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: Different pathways to failure. Nature Reviews. Endocrinology 16 (7):349–62. doi: 10.1038/s41574-020-0355-7.
- Fang, J. 2014. Bioavailability of anthocyanins. Drug Metabolism Reviews 46 (4):508–20. doi: 10.3109/03602532.2014.978080.
- Faria, A., I. Fernandes, S. Norberto, N. Mateus, and C. Calhau. 2014. Interplay between anthocyanins and gut microbiota. Journal of Agricultural and Food Chemistry 62 (29):6898–902. doi: 10.1021/jf501808a.
- Farrell, N. J., G. H. Norris, J. Ryan, C. M. Porter, C. Jiang, and C. N. Blesso. 2015. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. The British Journal of Nutrition 114 (8):1123–31. doi: 10.1017/S0007114515002962.
- Fu, Y. C., S. C. Yin, C. S. Chi, B. Hwang, and S. L. Hsu. 2006. Norepinephrine induces apoptosis in neonatal rat endothelial cells via a ROS-dependent JNK activation pathway. Apoptosis: An International Journal on Programmed Cell Death 11 (11):2053–63. doi: 10.1007/s10495-006-0192-8.
- Gaiz, A. A., S. Mosawy, N. Colson, and I. Singh. 2018. Potential of anthocyanin to prevent cardiovascular disease in diabetes. Alternative Therapies in Health and Medicine 24 (3):40–7.
- Grosso, G., U. Stepaniak, A. Micek, M. Kozela, D. Stefler, M. Bobak, and A. Pajak. 2017. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. The British Journal of Nutrition 118 (1):60–8. doi: 10.1017/S0007114517001805.
- Guo, H., J. Guo, X. Jiang, Z. Li, and W. Ling. 2012. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 50 (9):3040–7. doi: 10.1016/j.fct.2012.06.015.
- Guo, H., M. Xia, T. Zou, W. Ling, R. Zhong, and W. Zhang. 2012. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. The Journal of Nutritional Biochemistry 23 (4):349–60. doi: 10.1016/j.jnutbio.2010.12.013.
- Guo, X., B. Yang, J. Tan, J. Jiang, and D. Li. 2016. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. European Journal of Clinical Nutrition 70 (12):1360–7. doi: 10.1038/ejcn.2016.142.
- Hansson, J., L. Lind, J. Hulthe, and J. Sundström. 2009. Relations of serum MMP-9 and TIMP-1 levels to left ventricular measures and cardiovascular risk factors: A population-based study. European Journal of Cardiovascular Prevention & Rehabilitation 16 (3):297–303. doi: 10.1097/HJR.0b013e3283213108.
- He, B., J. Ge, P. Yue, X. Yue, R. Fu, J. Liang, and X. Gao. 2017. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chemistry 221:1671–7. doi: 10.1016/j.foodchem.2016.10.120.
- He, J., and M. M. Giusti. 2010. Anthocyanins: Natural colorants with health-promoting properties. Annual Review of Food Science and Technology 1:163–87. doi: 10.1146/annurev.food.080708.100754.
- He, Y., Y. Hu, X. Jiang, T. Chen, Y. Ma, S. Wu, J. Sun, R. Jiao, X. Li, L. Deng, et al. 2017. Cyanidin-3-O-glucoside inhibits the UVB-induced ROS/COX-2 pathway in HaCaT cells. Journal of Photochemistry and Photobiology. B, Biology 177:24–31. doi: 10.1016/j.jphotobiol.2017.10.006.
- Herrera-Balandrano, D. D., Z. Chai, R. P. Hutabarat, T. Beta, J. Feng, K. Ma, D. Li, and W. Huang. 2021. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biology. 46:102100. doi: 10.1016/j.redox.2021.102100.
- Heydemann, A. 2016. An overview of murine high fat diet as a model for type 2 diabetes mellitus. Journal of Diabetes Research 2016:2902351. doi: 10.1155/2016/2902351.
- Hidalgo, J., C. Flores, M. A. Hidalgo, M. Perez, A. Yañez, L. Quiñones, D. D. Caceres, and R. A. Burgos. 2014. Delphinol® standardized maqui berry extract reduces postprandial blood glucose increase in individuals with impaired glucose regulation by novel mechanism of sodium glucose cotransporter inhibition. Panminerva Medica 56 (2 Suppl 3):1–7.
- Hidalgo, M., M. J. Oruna-Concha, S. Kolida, G. E. Walton, S. Kallithraka, J. P. Spencer, and S. de Pascual-Teresa. 2012. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. Journal of Agricultural and Food Chemistry 60 (15):3882–90. doi: 10.1021/jf3002153.
- Hirose, A., T. Tanikawa, H. Mori, Y. Okada, and Y. Tanaka. 2010. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signaling pathway. FEBS Letters 584 (1):61–6. doi: 10.1016/j.febslet.2009.11.082.
- Hoggard, N., M. Cruickshank, K. M. Moar, C. Bestwick, J. J. Holst, W. Russell, and G. Horgan. 2013. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. Journal of Nutritional Science 2:e22. doi: 10.1017/jns.2013.16.
- Hou, D. X., K. Kai, J. J. Li, S. Lin, N. Terahara, M. Wakamatsu, M. Fujii, M. R. Young, and N. Colburn. 2004. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure-activity relationship and molecular mechanisms. Carcinogenesis 25 (1):29–36. doi: 10.1093/carcin/bgg184.
- Hsu, J. D., C. C. Wu, C. N. Hung, C. J. Wang, and H. P. Huang. 2016. Myrciaria cauliflora extract improves diabetic nephropathy via suppression of oxidative stress and inflammation in streptozotocin-nicotinamide mice. Journal of Food and Drug Analysis 24 (4):730–7. doi: 10.1016/j.jfda.2016.03.009.
- Huang, P. C., G. J. Wang, M. J. Fan, M. Asokan Shibu, Y. T. Liu, V. Padma Viswanadha, Y. L. Lin, C. H. Lai, Y. F. Chen, H. E. Liao, et al. 2017. Cellular apoptosis and cardiac dysfunction in STZ-induced diabetic rats attenuated by anthocyanins via activation of IGFI-R/PI3K/Akt survival signaling. Environmental Toxicology 32 (12):2471–80. doi: 10.1002/tox.22460.
- Huang, W., Z. Yan, D. Li, Y. Ma, J. Zhou, and Z. Sui. 2018. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxidative Medicine and Cellular Longevity 2018:1–10. doi: 10.1155/2018/1862462.
- Hwang, Y. P., J. H. Choi, E. H. Han, H. G. Kim, J. H. Wee, K. O. Jung, K. H. Jung, K. I. Kwon, T. C. Jeong, Y. C. Chung, et al. 2011. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutrition Research (New York, N.Y.) 31 (12):896–906. doi: 10.1016/j.nutres.2011.09.026.
- Iizuka, Y., A. Ozeki, T. Tani, and T. Tsuda. 2018. Blackcurrant extract ameliorates hyperglycemia in type 2 diabetic mice in association with increased basal secretion of glucagon-like peptide-1 and activation of AMP-activated protein kinase. Journal of Nutritional Science and Vitaminology 64 (4):258–64. doi: 10.3177/jnsv.64.258.
- Jacques, P. F., A. Cassidy, G. Rogers, J. J. Peterson, J. B. Meigs, and J. T. Dwyer. 2013. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. The Journal of Nutrition 143 (9):1474–80. doi: 10.3945/jn.113.177212.
- Jayaprakasam, B., L. K. Olson, R. E. Schutzki, M. H. Tai, and M. G. Nair. 2006. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). Journal of Agricultural and Food Chemistry 54 (1):243–8. doi: 10.1021/jf0520342.
- Jayaprakasam, B., S. K. Vareed, L. K. Olson, and M. G. Nair. 2005. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. Journal of Agricultural and Food Chemistry 53 (1):28–31. doi: 10.1021/jf049018+.
- Jeon, Y. D., S. H. Kang, K. H. Moon, J. H. Lee, D. G. Kim, W. Kim, J. S. Kim, B. Y. Ahn, and J. S. Jin. 2018. The effect of aronia berry on type 1 diabetes in vivo and in vitro. Journal of Medicinal Food 21 (3):244–53. doi: 10.1089/jmf.2017.3939.
- Ji, J., C. Zhang, X. Luo, L. Wang, R. Zhang, Z. Wang, D. Fan, H. Yang, and J. Deng. 2015. Effect of stay-green wheat, a novel variety of wheat in China, on glucose and lipid metabolism in high-fat diet induced type 2 diabetic rats. Nutrients 7 (7):5143–55. doi: 10.3390/nu7075143.
- Jiang, T., X. Shuai, J. Li, N. Yang, L. Deng, S. Li, Y. He, H. Guo, Y. Li, and J. He. 2020. Protein-bound anthocyanin compounds of purple sweet potato ameliorate hyperglycemia by regulating hepatic glucose metabolism in high-fat diet/streptozotocin-induced diabetic mice. Journal of Agricultural and Food Chemistry 68 (6):1596–608. doi: 10.1021/acs.jafc.9b06916.
- Jones, B., S. R. Bloom, T. Buenaventura, A. Tomas, and G. A. Rutter. 2018. Control of insulin secretion by GLP-1. Peptides 100:75–84. doi: 10.1016/j.peptides.2017.12.013.
- Jung, A. J., A. Sharma, S. H. Lee, S. J. Lee, J. H. Kim, and H. J. Lee. 2021. Efficacy of black rice extract on obesity in obese postmenopausal women: A 12-week randomized, double-blind, placebo-controlled preliminary clinical trial. Menopause (New York, NY) 28 (12):1391–9. doi: 10.1097/GME.0000000000001862.
- Jurgoński, A., J. Juśkiewicz, and Z. Zduńczyk. 2008. Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 63 (4):176–82. doi: 10.1007/s11130-008-0087-7.
- Kalt, W., A. Cassidy, L. R. Howard, R. Krikorian, A. J. Stull, F. Tremblay, and R. Zamora-Ros. 2020. Recent research on the health benefits of blueberries and their anthocyanins. Advances in Nutrition. 11 (2):224–36.
- Kang, M. K., J. Li, J. L. Kim, J. H. Gong, S. N. Kwak, J. H. Park, J. Y. Lee, S. S. Lim, and Y. H. Kang. 2012. Purple corn anthocyanins inhibit diabetes-associated glomerular monocyte activation and macrophage infiltration. American Journal of Physiology. Renal Physiology 303 (7):F1060–1069. doi: 10.1152/ajprenal.00106.2012.
- Kang, M. K., S. S. Lim, J. Y. Lee, K. M. Yeo, and Y. H. Kang. 2013. Anthocyanin-rich purple corn extract inhibit diabetes-associated glomerular angiogenesis. PloS One 8 (11):e79823. doi: 10.1371/journal.pone.0079823.
- Khan, R. M. M., Z. J. Y. Chua, J. C. Tan, Y. Yang, Z. Liao, and Y. Zhao. 2019. From pre-diabetes to diabetes: Diagnosis, treatments and translational research. Medicina 55 (9):546. doi: 10.3390/medicina55090546.
- Kianbakht, S., B. Abasi, and F. H. Dabaghian. 2013. Anti-hyperglycemic effect of Vaccinium arctostaphylos in type 2 diabetic patients: A randomized controlled trial. Complementary Medicine Research 20 (1):17–22. doi: 10.1159/000346607.
- Kim, H. J., K. A. Koo, W. S. Park, D. M. Kang, H. S. Kim, B. Y. Lee, Y. M. Goo, J. H. Kim, M. K. Lee, D. K. Woo, et al. 2020. Anti-obesity activity of anthocyanin and carotenoid extracts from color-fleshed sweet potatoes. Journal of Food Biochemistry:e13438. doi: 10.1111/jfbc.13438.
- Kim, J., C. S. Kim, Y. M. Lee, E. Sohn, K. Jo, and J. S. Kim. 2015. Vaccinium myrtillus extract prevents or delays the onset of diabetes–induced blood-retinal barrier breakdown. International Journal of Food Sciences and Nutrition 66 (2):236–42. doi: 10.3109/09637486.2014.979319.
- Kim, N. H., J. Jegal, Y. N. Kim, D. M. Chung, J. D. Heo, J. R. Rho, M. H. Yang, and E. J. Jeong. 2018. Antiobesity effect of fermented chokeberry extract in high-fat diet-induced obese mice. Journal of Medicinal Food 21 (11):1113–9. doi: 10.1089/jmf.2017.4124.
- Koh, E. S., J. H. Lim, M. Y. Kim, S. Chung, S. J. Shin, B. S. Choi, H. W. Kim, S. Y. Hwang, S. W. Kim, C. W. Park, et al. 2015. Anthocyanin-rich Seoritae extract ameliorates renal lipotoxicity via activation of AMP-activated protein kinase in diabetic mice. Journal of Translational Medicine 13:203. doi: 10.1186/s12967-015-0563-4.
- Kongthitilerd, P., T. Thilavech, M. Marnpae, W. Rong, S. Yao, S. Adisakwattana, H. Cheng, and T. Suantawee. 2022. Cyanidin-3-rutinoside stimulated insulin secretion through activation of L-type voltage-dependent Ca(2+) channels and the PLC-IP(3) pathway in pancreatic β-cells. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 146:112494. doi: 10.1016/j.biopha.2021.112494.
- Kurimoto, Y., Y. Shibayama, S. Inoue, M. Soga, M. Takikawa, C. Ito, F. Nanba, T. Yoshida, Y. Yamashita, H. Ashida, et al. 2013. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. Journal of Agricultural and Food Chemistry 61 (23):5558–64. doi: 10.1021/jf401190y.
- Kusunoki, M., D. Sato, K. Tsutsumi, H. Tsutsui, T. Nakamura, and Y. Oshida. 2015. Black soybean extract improves lipid profiles in fenofibrate-treated type 2 diabetics with postprandial hyperlipidemia. Journal of Medicinal Food 18 (6):615–8. doi: 10.1089/jmf.2014.3234.
- Laouali, N., T. Berrandou, A. J. Rothwell, S. Shah, D. El Fatouhi, F. R. Mancini, M. C. Boutron-Ruault, and G. Fagherazzi. 2020. Profiles of polyphenol intake and type 2 diabetes risk in 60,586 women followed for 20 years: Results from the E3N cohort study. Nutrients 12 (7):1934. doi: 10.3390/nu12071934.
- Lee, J. S., Y. R. Kim, I. G. Song, S. J. Ha, Y. E. Kim, N. I. Baek, and E. K. Hong. 2015. Cyanidin-3-glucoside isolated from mulberry fruit protects pancreatic β-cells against oxidative stress-induced apoptosis. International Journal of Molecular Medicine 35 (2):405–12. doi: 10.3892/ijmm.2014.2013.
- Lee, M., S. R. Sorn, Y. Park, and H. K. Park. 2016. Anthocyanin rich-black soybean testa improved visceral fat and plasma lipid profiles in overweight/obese Korean adults: A randomized controlled trial. Journal of Medicinal Food 19 (11):995–1003. doi: 10.1089/jmf.2016.3762.
- Lee, S. G., J. Chae, D. S. Kim, J. B. Lee, G. S. Kwon, T. K. Kwon, and J. O. Nam. 2021. Enhancement of the antiobesity and antioxidant effect of purple sweet potato extracts and enhancement of the effects by fermentation. Antioxidants 10 (6):888. doi: 10.3390/antiox10060888.
- Lee, S., K. I. Keirsey, R. Kirkland, Z. I. Grunewald, J. G. Fischer, and C. B. de La Serre. 2018. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet-fed rats. The Journal of Nutrition 148 (2):209–19. doi: 10.1093/jn/nxx027.
- Li, C., B. Yang, Z. Xu, E. Boivin, M. Black, W. Huang, B. Xu, P. Wu, B. Zhang, X. Li, et al. 2017. Protective effect of cyanidin-3-O-glucoside on neonatal porcine islets. The Journal of Endocrinology 235 (3):237–49. doi: 10.1530/JOE-17-0141.
- Li, D., P. Wang, Y. Luo, M. Zhao, and F. Chen. 2017. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Critical Reviews in Food Science and Nutrition 57 (8):1729–41. doi: 10.1080/10408398.2015.1030064.
- Li, D., Y. Zhang, Y. Liu, R. Sun, and M. Xia. 2015. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. The Journal of Nutrition 145 (4):742–8. doi: 10.3390/ijms222011076.
- Li, H., H. M. Park, H. S. Ji, J. Han, S. K. Kim, H. Y. Park, and T. S. Jeong. 2020. Phenolic-enriched blueberry-leaf extract attenuates glucose homeostasis, pancreatic β-cell function, and insulin sensitivity in high-fat diet-induced diabetic mice. Nutrition Research (New York, N.Y.) 73:83–96. doi: 10.1016/j.nutres.2019.09.005.
- Li, J., M. K. Kang, J. K. Kim, J. L. Kim, S. W. Kang, S. S. Lim, and Y. H. Kang. 2012. Purple corn anthocyanins retard diabetes-associated glomerulosclerosis in mesangial cells and db/db mice. European Journal of Nutrition 51 (8):961–73. doi: 10.1007/s00394-011-0274-4.
- Li, J., S. S. Lim, J. Y. Lee, J. K. Kim, S. W. Kang, J. L. Kim, and Y. H. Kang. 2012. Purple corn anthocyanins dampened high-glucose-induced mesangial fibrosis and inflammation: Possible renoprotective role in diabetic nephropathy. The Journal of Nutritional Biochemistry 23 (4):320–31. doi: 10.1016/j.jnutbio.2010.12.008.
- Liang, N., and D. D. Kitts. 2014. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules (Basel, Switzerland) 19 (11):19180–208. doi: 10.3390/molecules191119180.
- Ligthart, S., T. T. van Herpt, M. J. Leening, M. Kavousi, A. Hofman, B. H. Stricker, M. van Hoek, E. J. Sijbrands, O. H. Franco, and A. Dehghan. 2016. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: A prospective cohort study. Lancet Diabetes and Endocrinology. 4 (1):44–51. doi: 10.1016/S2213-8587(15)00362-9.
- Lila, M. A., B. Burton-Freeman, M. Grace, and W. Kalt. 2016. Unraveling anthocyanin bioavailability for human health. Annual Review of Food Science and Technology 7:375–93. doi: 10.1146/annurev-food-041715-033346.
- Lim, S. M., H. S. Lee, J. I. Jung, S. M. Kim, N. Y. Kim, T. S. Seo, J. S. Bae, and E. J. Kim. 2019. Cyanidin-3-O-galactoside-enriched Aronia melanocarpa extract attenuates weight gain and adipogenic pathways in high-fat diet-induced obese C57BL/6 mice. Nutrients 11 (5):1190. doi: 10.3390/nu11051190.
- Lin, B. W., C. C. Gong, H. F. Song, and Y. Y. Cui. 2017. Effects of anthocyanins on the prevention and treatment of cancer. British Journal of Pharmacology 174 (11):1226–43. doi: 10.1111/bph.13627.
- Lin, K., D. M. Lloyd-Jones, D. Li, and J. C. Carr. 2014. Quantitative imaging biomarkers for the evaluation of cardiovascular complications in type 2 diabetes mellitus. Journal of Diabetes and Its Complications 28 (2):234–42. doi: 10.1016/j.jdiacomp.2013.09.008.
- Liu, J., S. Tian, C. Xin, J. Liu, Q. Wang, Y. He, M. Liu, M. Fu, Y. Yang, and X. Cao. 2020. The Identification of Anthocyanins from Padus racemosa and its protective effects on H(2) O(2) -induced INS-1 cells damage and STZ-induced diabetes mice. Chemistry and Biodiversity 17 (11):e2000382.
- Liu, Y., D. Li, Y. Zhang, R. Sun, and M. Xia. 2014. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. American Journal of Physiology. Endocrinology and Metabolism 306 (8):E975–988. doi: 10.1152/ajpendo.00699.2013.
- Lontchi-Yimagou, E., E. Sobngwi, T. E. Matsha, and A. P. Kengne. 2013. Diabetes mellitus and inflammation. Current Diabetes Reports 13 (3):435–44. doi: 10.1007/s11892-013-0375-y.
- Lu, X., and C. Zhao. 2020. Exercise and type 1 diabetes. Advances in Experimental Medicine and Biology 1228:107–21.
- Luna-Vital, D. A., and E. Gonzalez de Mejia. 2018. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. Plos One 13 (7):e0200449. doi: 10.1371/journal.pone.0200449.
- Ma, Y., F. Chen, S. Yang, B. Chen, and J. Shi. 2018. Protocatechuic acid ameliorates high glucose-induced extracellular matrix accumulation in diabetic nephropathy. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 98:18–22. doi: 10.1016/j.biopha.2017.12.032.
- Ma, Z., B. Du, J. Li, Y. Yang, and F. Zhu. 2021. An insight into anti-inflammatory activities and inflammation related diseases of anthocyanins: A review of both in vivo and in vitro investigations. International Journal of Molecular Sciences 22 (20). doi: 10.1016/j.biopha.2017.12.032.
- Manach, C., G. Williamson, C. Morand, A. Scalbert, and C. Rémésy. 2005. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition 81 (1 Suppl):230s–42s. doi: 10.1093/ajcn/81.1.230S.
- Manucha, W. 2014. Mitochondria and oxidative stress participation in renal inflammatory process. Medicina 74 (3):254–8.
- Matsukawa, T., T. Inaguma, J. Han, M. O. Villareal, and H. Isoda. 2015. Cyanidin-3-glucoside derived from black soybeans ameliorate type 2 diabetes through the induction of differentiation of preadipocytes into smaller and insulin-sensitive adipocytes. The Journal of Nutritional Biochemistry 26 (8):860–7. doi: 10.1016/j.jnutbio.2015.03.006.
- Mattioli, R., A. Francioso, L. Mosca, and P. Silva. 2020. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 25 (17):3809. doi: 10.3390/molecules25173809.
- Maya-Monteiro, C. M., and P. T. Bozza. 2008. Leptin and mTOR: Partners in metabolism and inflammation. Cell Cycle (Georgetown, TX) 7 (12):1713–7. doi: 10.4161/cc.7.12.6157.
- Mayans, L. 2015. Metabolic syndrome: Insulin resistance and prediabetes. FP Essent 435:11–6.
- Mhya, D. H., A. Nuhu, and M. J. N. R. f H. H. Mankilik. 2021. In-silico discovery of antidiabetic drug potential of Balanites aegyptiaca leaf’s phenolic compounds. Natural Resources for Human Health 1 (2):91–97. doi: 10.53365/nrfhh/142375.
- Miki, A., C. Ricordi, Y. Sakuma, T. Yamamoto, R. Misawa, A. Mita, R. D. Molano, N. D. Vaziri, A. Pileggi, and H. Ichii. 2018. Divergent antioxidant capacity of human islet cell subsets: A potential cause of beta-cell vulnerability in diabetes and islet transplantation. PloS One 13 (5):e0196570.
- Millar, C. L., Q. Duclos, and C. N. Blesso. 2017. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Advances in Nutrition (Bethesda, MD) 8 (2):226–39. doi: 10.3945/an.116.014050.
- Moazen, S., R. Amani, A. Homayouni Rad, H. Shahbazian, K. Ahmadi, and M. Taha Jalali. 2013. Effects of freeze-dried strawberry supplementation on metabolic biomarkers of atherosclerosis in subjects with type 2 diabetes: A randomized double-blind controlled trial. Annals of Nutrition and Metabolism 63 (3):256–64. doi: 10.1159/000356053.
- Montanari, E., C. Gonelle-Gispert, J. D. Seebach, M. F. Knoll, R. Bottino, and L. H. Bühler. 2019. Immunological aspects of allogeneic pancreatic islet transplantation: A comparison between mouse and human. Transplant International: Official Journal of the European Society for Organ Transplantation 32 (9):903–12. doi: 10.1111/tri.13445.
- Muraki, I., F. Imamura, J. E. Manson, F. B. Hu, W. C. Willett, R. M. van Dam, and Q. Sun. 2013. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ 347 (aug28 1):f5001–f5001. doi: 10.1136/bmj.f5001.
- Mussa, B. M., A. Srivastava, A. Al-Habshi, A. K. Mohammed, R. Halwani, and S. Abusnana. 2021. Inflammatory biomarkers levels in T2DM Emirati patients with diabetic neuropathy. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 14:3389–97. doi: 10.2147/DMSO.S319863.
- Mykkänen, O. T., A. Huotari, K. H. Herzig, T. W. Dunlop, H. Mykkänen, and P. V. Kirjavainen. 2014. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PloS One 9 (12):e114790. doi: 10.1371/journal.pone.0114790.
- Nasri, S., M. Roghani, T. Baluchnejadmojarad, T. Rabani, and M. Balvardi. 2011. Vascular mechanisms of cyanidin-3-glucoside response in streptozotocin-diabetic rats. Pathophysiology: The Official Journal of the International Society for Pathophysiology 18 (4):273–8. doi: 10.1016/j.pathophys.2011.03.001.
- Nemes, A., J. R. Homoki, R. Kiss, C. Hegedűs, D. Kovács, B. Peitl, F. Gál, L. Stündl, Z. Szilvássy, and J. Remenyik. 2019. Effect of anthocyanin-rich tart cherry extract on inflammatory mediators and adipokines involved in type 2 diabetes in a high fat diet induced obesity mouse model. Nutrients 11 (9):1966. doi: 10.3390/nu11091966.
- Nikbakht, E., I. Singh, J. Vider, L. T. Williams, L. Vugic, A. Gaiz, A. R. Kundur, and N. Colson. 2021. Potential of anthocyanin as an anti-inflammatory agent: A human clinical trial on type 2 diabetic, diabetic at-risk and healthy adults. Inflammation Research: Official Journal of the European Histamine Research Society. [et al.] 70 (3):275–84. doi: 10.1007/s00011-021-01438-1.
- Nizamutdinova, I. T., Y. C. Jin, J. I. Chung, S. C. Shin, S. J. Lee, H. G. Seo, J. H. Lee, K. C. Chang, and H. J. Kim. 2009. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Molecular Nutrition & Food Research 53 (11):1419–29. doi: 10.1002/mnfr.200800526.
- Nomi, Y., K. Iwasaki-Kurashige, and H. Matsumoto. 2019. Therapeutic effects of anthocyanins for vision and eye health. Molecules 24 (18):3311. doi: 10.3390/molecules24183311.
- Noratto, G. D., N. N. Lage, B. P. Chew, S. U. Mertens-Talcott, S. T. Talcott, and M. L. Pedrosa. 2018. Non-anthocyanin phenolics in cherry (Prunus avium L.) modulate IL-6, liver lipids and expression of PPARδ and LXRs in obese diabetic (db/db) mice. Food Chemistry 266:405–14. doi: 10.1016/j.foodchem.2018.06.020.
- Novoselova, E. G., O. V. Glushkova, M. O. Khrenov, S. M. Lunin, T. V. Novoselova, and S. B. Parfenuyk. 2021. Role of innate immunity and oxidative stress in the development of type 1 diabetes mellitus. Peroxiredoxin 6 as a new anti-diabetic agent. Biochemistry. Biokhimiia 86 (12):1579–89. doi: 10.1134/S0006297921120075.
- Oliveira, P. S., M. Gazal, N. P. Flores, A. R. Zimmer, V. C. Chaves, F. H. Reginatto, M. P. Kaster, R. G. Tavares, R. M. Spanevello, C. L. Lencina, et al. 2017. Vaccinium virgatum fruit extract as an important adjuvant in biochemical and behavioral alterations observed in animal model of metabolic syndrome. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 88:939–47. doi: 10.1016/j.biopha.2017.01.121.
- Oyama, L. M., F. P. Silva, J. Carnier, D. A. de Miranda, A. B. Santamarina, E. B. Ribeiro, C. M. Oller do Nascimento, and V. V. de Rosso. 2016. Juçara pulp supplementation improves glucose tolerance in mice. Diabetology & Metabolic Syndrome 8:8. doi: 10.1186/s13098-015-0122-4.
- Palacios, O. M., M. Kramer, and K. C. Maki. 2019. Diet and prevention of type 2 diabetes mellitus: Beyond weight loss and exercise. Expert Review of Endocrinology & Metabolism 14 (1):1–12. doi: 10.1080/17446651.2019.1554430.
- Panchamoorthy, R., and N. Vel. 2021. Herbal spices-based therapeutics for diabetic patients with COVID-19 infection: A review. Natural Resources for Human Health 2 (1):32–51.
- Park, E., I. Edirisinghe, H. Wei, L. P. Vijayakumar, K. Banaszewski, J. C. Cappozzo, and B. Burton-Freeman. 2016. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Molecular Nutrition & Food Research 60 (5):1099–109. doi: 10.1002/mnfr.201500845.
- Park, M., J. H. Yoo, Y. S. Lee, and H. J. Lee. 2019. Lonicera caerulea extract attenuates non-alcoholic fatty liver disease in free fatty acid-induced HepG2 hepatocytes and in high fat diet-fed mice. Nutrients 11 (3):494. doi: 10.3390/nu11030494.
- Park, S., S. Kang, D. Y. Jeong, S. Y. Jeong, J. J. Park, and H. S. Yun. 2015. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes & Nutrition 10 (2):455. doi: 10.1007/s12263-015-0455-5.
- Petersmann, A., D. Müller-Wieland, U. A. Müller, R. Landgraf, M. Nauck, G. Freckmann, L. Heinemann, and E. Schleicher. 2019. Definition, classification and diagnosis of diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes: Official Journal, German Society of Endocrinology [and] German Diabetes Association 127 (S 01):S1–s7. doi: 10.1055/a-1018-9078.
- Preedy, V. R. 2014. Handbook of nutrition, diet, and the eye. London: Academic Press.
- Qi, C., X. Mao, Z. Zhang, and H. Wu. 2017. Classification and differential diagnosis of diabetic nephropathy. Journal of Diabetes Research 2017:1–7. doi: 10.1155/2017/8637138.
- Qin, B., and R. A. Anderson. 2012. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich diet. The British Journal of Nutrition 108 (4):581–7. doi: 10.1017/S000711451100599X.
- Qin, Y., Q. Zhai, Y. Li, M. Cao, Y. Xu, K. Zhao, and T. Wang. 2018. Cyanidin-3-O-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 103:1223–30. doi: 10.1016/j.biopha.2018.04.137.
- Reis, J. F., V. V. Monteiro, R. de Souza Gomes, M. M. do Carmo, G. V. da Costa, P. C. Ribera, and M. C. Monteiro. 2016. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. Journal of Translational Medicine 14 (1):315. doi: 10.1186/s12967-016-1076-5.
- Ren, S., R. Jiménez-Flores, and M. M. Giusti. 2021. The interactions between anthocyanin and whey protein: A review. Comprehensive Reviews in Food Science and Food Safety 20 (6):5992–6011. doi: 10.1111/1541-4337.12854.
- Rickels, M. R., and R. P. Robertson. 2019. Pancreatic islet transplantation in humans: Recent progress and future directions. Endocrine Reviews 40 (2):631–68. doi: 10.1210/er.2018-00154.
- Rienks, J., J. Barbaresko, K. Oluwagbemigun, M. Schmid, and U. Nöthlings. 2018. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. The American Journal of Clinical Nutrition 108 (1):49–61. doi: 10.1093/ajcn/nqy083.
- Rojo, L. E., D. Ribnicky, S. Logendra, A. Poulev, P. Rojas-Silva, P. Kuhn, R. Dorn, M. H. Grace, M. A. Lila, and I. Raskin. 2012. In vitro and in vivo anti-diabetic effects of anthocyanins from maqui berry (Aristotelia chilensis). Food Chemistry 131 (2):387–96. doi: 10.1016/j.foodchem.2011.08.066.
- Roopchand, D. E., P. Kuhn, L. E. Rojo, M. A. Lila, and I. Raskin. 2013. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacological Research 68 (1):59–67. doi: 10.1016/j.phrs.2012.11.008.
- Saeedi, P., I. Petersohn, P. Salpea, B. Malanda, S. Karuranga, N. Unwin, S. Colagiuri, L. Guariguata, A. A. Motala, K. Ogurtsova, et al. 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Research and Clinical Practice 157:107843. doi: 10.1016/j.diabres.2019.107843.
- Saito, T., M. Nishida, M. Saito, A. Tanabe, T. Eitsuka, S. H. Yuan, N. Ikekawa, and H. Nishida. 2016. The fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms improves insulin resistance and hepatic lipid accumulation by modulation of liver adenosine monophosphate-activated protein kinase activity and lipogenic gene expression in high-fat diet-fed obese mice. Nutrition Research 36 (10):1090–7.
- Salehi, B., A. Ata, N. V Anil Kumar, F. Sharopov, K. Ramírez-Alarcón, A. Ruiz-Ortega, S. Abdulmajid Ayatollahi, P. V. Tsouh Fokou, F. Kobarfard, Z. Amiruddin Zakaria, et al. 2019. Antidiabetic potential of medicinal plants and their active components. Biomolecules 9 (10):551. [ Mismatch] doi: 10.3390/biom9100551.
- Sanchez-Niño, M. D., A. B. Sanz, C. Lorz, A. Gnirke, M. P. Rastaldi, V. Nair, J. Egido, M. Ruiz-Ortega, M. Kretzler, and A. Ortiz. 2010. BASP1 promotes apoptosis in diabetic nephropathy. Journal of the American Society of Nephrology: JASN 21 (4):610–21. doi: 10.1681/ASN.2009020227.
- Sarikaphuti, A., T. Nararatwanchai, T. Hashiguchi, T. Ito, S. Thaworanunta, K. Kikuchi, Y. Oyama, I. Maruyama, and S. Tancharoen. 2013. Preventive effects of Morus alba L. anthocyanins on diabetes in Zucker diabetic fatty rats. Experimental and Therapeutic Medicine 6 (3):689–95. doi: 10.3892/etm.2013.1203.
- Sasaki, R., N. Nishimura, H. Hoshino, Y. Isa, M. Kadowaki, T. Ichi, A. Tanaka, S. Nishiumi, I. Fukuda, H. Ashida, et al. 2007. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochemical Pharmacology 74 (11):1619–27.
- Semaming, Y., S. Kumfu, P. Pannangpetch, S. C. Chattipakorn, and N. Chattipakorn. 2014. Protocatechuic acid exerts a cardioprotective effect in type 1 diabetic rats. The Journal of Endocrinology 223 (1):13–23. doi: 10.1530/JOE-14-0273.
- Seymour, E. M., I. I. Tanone, D. E. Urcuyo-Llanes, S. K. Lewis, A. Kirakosyan, M. G. Kondoleon, P. B. Kaufman, and S. F. Bolling. 2011. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. Journal of Medicinal Food 14 (12):1511–8. doi: 10.1089/jmf.2010.0292.
- Shapiro, A. M., M. Pokrywczynska, and C. Ricordi. 2017. Clinical pancreatic islet transplantation. Nature Reviews. Endocrinology 13 (5):268–77. doi: 10.1038/nrendo.2016.178.
- Shen, Y., N. Zhang, J. Tian, G. Xin, L. Liu, X. Sun, and B. Li. 2022. Advanced approaches for improving bioavailability and controlled release of anthocyanins. Journal of Controlled Release: Official Journal of the Controlled Release Society 341:285–99. doi: 10.1016/j.jconrel.2021.11.031.
- Silva, S., E. M. Costa, C. Calhau, R. M. Morais, and M. E. Pintado. 2017. Anthocyanin extraction from plant tissues: A review. Critical Reviews in Food Science and Nutrition 57 (14):3072–83. doi: 10.1080/10408398.2015.1087963.
- Silveira, J. Q., G. K. Dourado, and T. B. Cesar. 2015. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. International Journal of Food Sciences and Nutrition 66 (7):830–6. doi: 10.3109/09637486.2015.1093610.
- Singh, R. P., M. J. Elman, S. K. Singh, A. E. Fung, and I. Stoilov. 2019. Advances in the treatment of diabetic retinopathy. Journal of Diabetes and Its Complications 33 (12):107417. doi: 10.1016/j.jdiacomp.2019.107417.
- Solverson, P. M., T. R. Henderson, H. Debelo, M. G. Ferruzzi, D. J. Baer, and J. A. Novotny. 2019. An anthocyanin-rich mixed-berry intervention may improve insulin sensitivity in a randomized trial of overweight and obese adults. Nutrients 11 (12):2876. doi: 10.3390/nu11122876.
- Solverson, P. M., W. V. Rumpler, J. L. Leger, B. W. Redan, M. G. Ferruzzi, D. J. Baer, T. W. Castonguay, and J. A. Novotny. 2018. Blackberry feeding increases fat oxidation and improves insulin sensitivity in overweight and obese males. Nutrients 10 (8):1048. doi: 10.3390/nu10081048.
- Song, H., X. Shen, F. Wang, Y. Li, and X. Zheng. 2021. Black current anthocyanins improve lipid metabolism and modulate gut microbiota in high-fat diet-induced obese mice. Molecular Nutrition & Food Research 65 (6):e2001090. doi: 10.1002/mnfr.202001090.
- Song, H., X. Shen, Y. Zhou, and X. Zheng. 2021. Black rice anthocyanins alleviate hyperlipidemia, liver steatosis and insulin resistance by regulating lipid metabolism and gut microbiota in obese mice. Food & Function 12 (20):10160–70. doi: 10.1039/d1fo01394g.
- Song, Y., L. Huang, and J. Yu. 2016. Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. Journal of Neuroimmunology 301:1–6. doi: 10.1016/j.jneuroim.2016.11.001.
- Soon, Y. Y., and B. K. Tan. 2002. Evaluation of the hypoglycemic and anti-oxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats. Singapore Medical Journal 43 (2):077–85.
- Stevens, M., C. R. Neal, E. C. Craciun, M. Dronca, S. J. Harper, and S. Oltean. 2019. The natural drug DIAVIT is protective in a type II mouse model of diabetic nephropathy. PloS One 14 (3):e0212910. doi: 10.1371/journal.pone.0212910.
- Strugała, P., O. Dzydzan, I. Brodyak, A. Z. Kucharska, P. Kuropka, M. Liuta, K. Kaleta-Kuratewicz, A. Przewodowska, D. Michałowska, J. Gabrielska, et al. 2019. Antidiabetic and antioxidative potential of the blue congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules 24 (17):3126. doi: 10.3390/molecules24173126.
- Stull, A. J., K. C. Cash, W. D. Johnson, C. M. Champagne, and W. T. Cefalu. 2010. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. The Journal of Nutrition 140 (10):1764–8. doi: 10.3945/jn.110.125336.
- Suantawee, T., S. T. Elazab, W. H. Hsu, S. Yao, H. Cheng, and S. Adisakwattana. 2017. Cyanidin stimulates insulin secretion and pancreatic β-cell gene expression through activation of l-type voltage-dependent Ca(2+) channels. Nutrients 9 (8):814. doi: 10.3390/nu9080814.
- Sun, H., P. Zhang, Y. Zhu, Q. Lou, and S. He. 2018. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweet potato (Ipomoea batatas (L.) Lam.). Scientific Reports 8 (1):5018. doi: 10.1038/s41598-018-23397-0.
- Takikawa, M., S. Inoue, F. Horio, and T. Tsuda. 2010. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. The Journal of Nutrition 140 (3):527–33. doi: 10.3945/jn.109.118216.
- Thorens, B. 2014. Neural regulation of pancreatic islet cell mass and function. Diabetes, Obesity & Metabolism 16 Suppl 1:87–95. doi: 10.1111/dom.12346.
- Tian, J. L., X. Si, C. Shu, Y. H. Wang, H. Tan, Z. H. Zang, W. J. Zhang, X. Xie, Y. Chen, and B. Li. 2022. Synergistic effects of combined anthocyanin and metformin treatment for hyperglycemia in vitro and in vivo. Journal of Agricultural and Food Chemistry 70 (4):1182–95. doi: 10.1021/acs.jafc.1c07799.
- Tian, L., H. Ning, W. Shao, Z. Song, Y. Badakhshi, W. Ling, B. B. Yang, P. L. Brubaker, and T. Jin. 2020. Dietary cyanidin-3-glucoside attenuates high-fat-diet-induced body-weight gain and impairment of glucose tolerance in mice via effects on the hepatic hormone FGF21. The Journal of Nutrition 150 (8):2101–11. doi: 10.1093/jn/nxaa140.
- Tian, L., Y. Tan, G. Chen, G. Wang, J. Sun, S. Ou, W. Chen, and W. Bai. 2019. Metabolism of anthocyanins and consequent effects on the gut microbiota. Critical Reviews in Food Science and Nutrition 59 (6):982–91. doi: 10.1080/10408398.2018.1533517.
- Tsuda, T. 2017. Prevention and treatment of diabetes using polyphenols via activation of AMP-activated protein kinase and stimulation of glucagon-like peptide-1 secretion. In Recent advances in polyphenol research, vol. 5, 206–25. Oxford: Blackwell publishing.
- Tsuda, T., F. Horio, K. Uchida, H. Aoki, and T. Osawa. 2003. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. The Journal of Nutrition 133 (7):2125–30. doi: 10.1093/jn/133.7.2125.
- Valente, A. J., T. Yoshida, J. D. Gardner, N. Somanna, P. Delafontaine, and B. Chandrasekar. 2012. Interleukin-17A stimulates cardiac fibroblast proliferation and migration via negative regulation of the dual-specificity phosphatase MKP-1/DUSP-1. Cellular Signalling 24 (2):560–8. doi: 10.1016/j.cellsig.2011.10.010.
- Vilhena, R. O., I. D. Figueiredo, A. M. Baviera, D. B. Silva, B. M. Marson, J. A. Oliveira, R. G. Peccinini, I. K. Borges, and R. Pontarolo. 2020. Antidiabetic activity of Musa x paradisiaca extracts in streptozotocin-induced diabetic rats and chemical characterization by HPLC-DAD-MS. Journal of Ethnopharmacology 254:112666. doi: 10.1016/j.jep.2020.112666.
- Wada, J., and H. Makino. 2013. Inflammation and the pathogenesis of diabetic nephropathy. Clinical Science (London, England : 1979) 124 (3):139–52. doi: 10.1042/CS20120198.
- Wang, X., Y. Y. Ouyang, J. Liu, and G. Zhao. 2014. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. The British Journal of Nutrition 111 (1):1–11. doi: 10.1017/S000711451300278X.
- Watanabe, M., and J. Ayugase. 2010. Effects of buckwheat sprouts on plasma and hepatic parameters in type 2 diabetic DB/DB mice. Journal of Food Science 75 (9):H294–299. doi: 10.1111/j.1750-3841.2010.01853.x.
- Wedick, N. M., A. Pan, A. Cassidy, E. B. Rimm, L. Sampson, B. Rosner, W. Willett, F. B. Hu, Q. Sun, and R. M. van Dam. 2012. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. The American Journal of Clinical Nutrition 95 (4):925–33. doi: 10.3945/ajcn.111.028894.
- Wei, J., H. Wu, H. Zhang, F. Li, S. Chen, B. Hou, Y. Shi, L. Zhao, and H. Duan. 2018. Anthocyanins inhibit high glucose-induced renal tubular cell apoptosis caused by oxidative stress in DB/DB mice. International Journal of Molecular Medicine 41 (3):1608–18. doi: 10.3892/ijmm.2018.3378.
- Williamson, G. 2013. Possible effects of dietary polyphenols on sugar absorption and digestion. Molecular Nutrition & Food Research 57 (1):48–57. doi: 10.1002/mnfr.201200511.
- Winter, A. N., and P. C. Bickford. 2019. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants (Basel) 8 (9):333. doi: 10.3390/antiox8090333.
- Wu, C. C., C. N. Hung, Y. C. Shin, C. J. Wang, and H. P. Huang. 2016. Myrciaria cauliflora extracts attenuate diabetic nephropathy involving the Ras signaling pathway in streptozotocin/nicotinamide mice on a high fat diet. Journal of Food and Drug Analysis 24 (1):136–46. doi: 10.1016/j.jfda.2015.10.001.
- Wu, T., L. Yang, X. Guo, M. Zhang, R. Liu, and W. Sui. 2018. Raspberry anthocyanin consumption prevents diet-induced obesity by alleviating oxidative stress and modulating hepatic lipid metabolism. Food & Function 9 (4):2112–20. doi: 10.1039/c7fo02061a.
- Wu, T., Z. Jiang, J. Yin, H. Long, and X. Zheng. 2016. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice. International Journal of Food Sciences and Nutrition 67 (3):257–64. doi: 10.3109/09637486.2016.1146235.
- Xu, H., J. Luo, J. Huang, and Q. Wen. 2018. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 97 (19):e0686. doi: 10.1097/MD.0000000000010686.
- Xu, M., K. A. Bower, S. Wang, J. A. Frank, G. Chen, M. Ding, S. Wang, X. Shi, Z. Ke, and J. Luo. 2010. Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Molecular Cancer 9:285. doi: 10.1186/1476-4598-9-285.
- Yamane, T., M. Kozuka, D. Konda, Y. Nakano, T. Nakagaki, I. Ohkubo, and H. Ariga. 2016. Improvement of blood glucose levels and obesity in mice given aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. The Journal of Nutritional Biochemistry 31:106–12. doi: 10.1016/j.jnutbio.2016.02.004.
- Yan, F., G. Dai, and X. Zheng. 2016. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. The Journal of Nutritional Biochemistry 36:68–80. doi: 10.1016/j.jnutbio.2016.07.004.
- Yang, L., W. Ling, Y. Qiu, Y. Liu, L. Wang, J. Yang, C. Wang, and J. Ma. 2020. Anthocyanins increase serum adiponectin in newly diagnosed diabetes but not in prediabetes: A randomized controlled trial. Nutrition & Metabolism 17:78. doi: 10.1186/s12986-020-00498-0.
- Yang, L., W. Ling, Y. Yang, Y. Chen, Z. Tian, Z. Du, J. Chen, Y. Xie, Z. Liu, and L. Yang. 2017. Role of purified anthocyanins in improving cardiometabolic risk factors in Chinese men and women with prediabetes or early untreated diabetes-a randomized controlled trial. Nutrients 9 (10):1104. doi: 10.3390/nu9101104.
- Yang, L., Y. Qiu, W. Ling, Z. Liu, L. Yang, C. Wang, X. Peng, L. Wang, and J. Chen. 2021. Anthocyanins regulate serum adipsin and visfatin in patients with prediabetes or newly diagnosed diabetes: A randomized controlled trial. European Journal of Nutrition 60 (4):1935–44. doi: 10.1007/s00394-020-02379-x.
- Yang, P., C. Yuan, H. Wang, F. Han, Y. Liu, L. Wang, and Y. Liu. 2018. Stability of anthocyanins and their degradation products from cabernet sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules 23 (2):354. doi: 10.3390/molecules23020354.
- Ye, X., W. Chen, P. Tu, R. Jia, Y. Liu, Q. Tang, C. Chen, C. Yang, X. Zheng, and Q. Chu. 2022. Antihyperglycemic effect of an anthocyanin, cyanidin-3-O-glucoside, is achieved by regulating GLUT-1 via the Wnt/β-catenin-WISP1 signaling pathway. Food & Function 13 (8):4612–23. doi: 10.1039/D1FO03730G.
- Ye, X., W. Chen, P. Tu, R. Jia, Y. Liu, Y. Li, Q. Tang, X. Zheng, and Q. Chu. 2021. Food-derived cyanidin-3-O-glucoside alleviates oxidative stress: Evidence from the islet cell line and diabetic DB/DB mice. Food & Function 12 (22):11599–610. doi: 10.1039/d1fo02385c.
- Yue, E., Y. Yu, X. Wang, B. Liu, Y. Bai, and B. Yang. 2020. Anthocyanin protects cardiac function and cardiac fibroblasts from high-glucose induced inflammation and myocardial fibrosis by inhibiting IL-17. Frontiers in Pharmacology 11:593633.
- Zeynaloo, E., L. D. Stone, E. Dikici, C. Ricordi, S. K. Deo, L. G. Bachas, S. Daunert, and G. Lanzoni. 2022. Delivery of therapeutic agents and cells to pancreatic islets: Towards a new era in the treatment of diabetes. Molecular Aspects of Medicine 83:101063. doi: 10.1016/j.mam.2021.101063.
- Zhang, B., M. Buya, W. Qin, C. Sun, H. Cai, Q. Xie, B. Xu, and Y. Wu. 2013. Anthocyanins from Chinese bayberry extract activate transcription factor Nrf2 in β cells and negatively regulate oxidative stress-induced autophagy. Journal of Agricultural and Food Chemistry 61 (37):8765–72. doi: 10.1021/jf4012399.
- Zhang, B., M. Kang, Q. Xie, B. Xu, C. Sun, K. Chen, and Y. Wu. 2011. Anthocyanins from Chinese bayberry extract protect β cells from oxidative stress-mediated injury via HO-1 upregulation. Journal of Agricultural and Food Chemistry 59 (2):537–45. doi: 10.1021/jf1035405.
- Zhang, W., H. Liu, M. Rojas, R. W. Caldwell, and R. B. Caldwell. 2011. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy 3 (5):609–28. doi: 10.2217/imt.11.24.
- Zhao, C. L., Y. Q. Yu, Z. J. Chen, G. S. Wen, F. G. Wei, Q. Zheng, C. D. Wang, and X. L. Xiao. 2017. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chemistry 214:119–28. doi: 10.1016/j.foodchem.2016.07.073.
- Zhao, F., X. Gao, X. Ge, J. Cui, and X. Liu. 2021. Cyanidin-3-o-glucoside (C3G) inhibits vascular leakage regulated by microglial activation in early diabetic retinopathy and neovascularization in advanced diabetic retinopathy. Bioengineered 12 (2):9266–78. doi: 10.1080/21655979.2021.1996512.
- Zheng, H. X., S. S. Qi, J. He, C. Y. Hu, H. Han, H. Jiang, and X. S. Li. 2020. Cyanidin-3-glucoside from black rice ameliorates diabetic nephropathy via reducing blood glucose, suppressing oxidative stress and inflammation, and regulating transforming growth factor β1/smad expression. Journal of Agricultural and Food Chemistry 68 (15):4399–410. doi: 10.1021/acs.jafc.0c00680.
- Zhu, Y., H. Sun, S. He, Q. Lou, M. Yu, M. Tang, and L. Tu. 2018. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. Plos One 13 (4):e0195754. doi: 10.1371/journal.pone.0195754.
- Zou, W., C. Zhang, X. Gu, X. Li, and H. Zhu. 2021. Metformin in combination with malvidin prevents progression of non-alcoholic fatty liver disease via improving lipid and glucose metabolisms, and inhibiting inflammation in type 2 diabetes rats. Drug Design, Development and Therapy 15:2565–76. doi: 10.2147/DDDT.S307257.