?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Tetracyclines are a group of antibiotic substances largely administered through medicated feed to control diseases in food-producing animals. Fine dosing of antibiotics contained in medicated feed is crucial for the success of the treatment as well as minimising potential threats such as the spread of antimicrobial resistance and the transfer of antibiotic residues in food. A rapid analytical method based on HPLC with diode array detection (HPLC–DAD) was developed to quantify oxytetracycline, chlortetracycline and doxycycline in medicated feed. The reported method underwent in-house validation and was found to be suitable for the quantification of three target tetracyclines within the concentration range of 40–1000 mg kg−1 in official routine analysis. The method was applied to 103 official samples in the framework of the Italian National Plan on animal feed during the years 2021–2023 and nine non-compliant concentrations were identified in swine and fish feed samples.
Introduction
Antibiotics are an effective and widely used tool to treat and prevent infectious diseases in farmed animals. Particularly, in intensive production systems, where pathogens may easily spread from one animal to another, the use of antibiotic substances has an important role in controlling diseases aiming to improve animal health and avoid economic losses (Mehdi et al. Citation2018). Medicated feed is one of the possible routes for oral administration of antibiotic drugs to the animals allowing the farmer to perform the concurrent treatment of large groups of animals. Antibiotic treatment through medicated feed is available under veterinary prescription, which defines the specific compound to be administered and the correct dosage (Dewell et al. Citation2022). However, during the group administration of the medicated feed the oral dosage may vary between each medication for one single treated animal as well as between animals due to multiple factors, such as individual health conditions or inhomogeneous distribution and stability of the medicinal compounds in the feed mixture (Vandael et al. Citation2019; Georgaki et al. Citation2023). Moreover, cross-contamination and carry-over phenomena with non-medicated feed may occur, leading to antibiotic residues in food of animal origin (Stolker et al. Citation2013; Santos-Santórum Suárez et al. Citation2023). All these issues contribute to the spread of antibiotic resistance, which nowadays is one of the major health concerns worldwide (Kahn Citation2017). In 2021, EFSA assessed the concentrations of antimicrobials resulting from cross-contamination in non-target feed for food-producing animals. The assessment aimed to determine the specific concentrations below which there would be no selection of resistance in microbial agents (Koutsoumanis et al. Citation2021). Tetracyclines are among the few substances for which the assessment was provided. The Feed Antimicrobial Resistance Selection Concentration calculated for these molecules is extremely low, at around a few micrograms per kilogram (Koutsoumanis et al. Citation2021).
Antibiotics belonging to the group of tetracyclines are often used for the treatment of bacterial infections in food-producing animal species (cattle, poultry, swine and aquaculture) due to their broad-spectrum activity against both Gram-positive and Gram-negative bacteria and due to their economic advantages (Apley et al. Citation2012; Mehdi et al. Citation2018). First-generation tetracyclines, such as chlortetracycline, oxytetracycline and tetracycline, are chosen for livestock treatment. However, the semi-synthetic second-generation tetracycline derivative doxycycline, which has a long history of use in human medicine, is also indicated for veterinary use (Granados-Chinchilla and Rodríguez Citation2017). Tetracyclines are listed in the Category D – ‘Prudence’ (lowest risk category) in the categorisation of veterinary antibiotics used in animals redacted by European Medicine Agency (EMA) and are the first line treatments in animals, while group treatments may be applied restricted to feasible situations ([EMA] European Medicine Agency Citation2019). In Italy, feed premixes containing chlortetracycline, oxytetracycline and doxycycline are approved for therapeutic use in food-producing animals (Ministero della Salute Citation2017-2024). Accurate drug dosage in animal feed is essential for effective treatment. Sub-therapeutic concentrations of antibiotic substances, including tetracyclines, have been historically used for growth promotion purposes in food-producing animals with important consequences on the spread of antimicrobial resistance and were banned in EU since 2006 (Brown et al. Citation2017). Veterinary treatments may result in traces of antibiotic drugs remaining in food of animal origin posing a potential risk to human health (Ghimpețeanu et al. Citation2022). Maximum residue limits for antibiotic drugs, including tetracyclines, in food of animal origin are subject to the Regulation (EU) 37/2010 (European Commission Citation2010). To minimise the transfer of veterinary drug residues in food, good practices for responsible use of antibiotics should be followed. Along with observing withdrawal periods, ensuring the correct dosage of drugs is essential for this purpose. Currently, the manufacturing, placing on the market and use of medicated feed in the EU are regulated under Regulation 2019/4 (European Parliament and Council Citation2019). All Member States are required to carry out official controls on application of food and feed as per Regulation (EU) 2017/625 (European Commission Citation2017). In Italy, the official controls ensuring the feed quality are performed in the framework of the three-year National Plans on animal feed through the monitoring of veterinary drugs cross-contamination and carry-over and of the correct dosing of active ingredients in medicated feed (Ministero della Salute Citation2021).
The highly sensitive analytical determination of antibiotic substances, including tetracyclines, in food is achieved by LC–MS/MS which gives optimal performance for the quantification of the veterinary drug residues at levels of µg kg−1 (Desmarchelier et al. Citation2018). The same analytical approach is applied to feedstuff for determination of antibiotic residues in cross-contamination and carry-over analyses (Gavilán et al. Citation2015; Patyra and Kwiatek Citation2017; Jank et al. Citation2018). However, tetracyclines are susceptible to matrix effect in MS analysis and, given the complexity of the feedstuff matrices, the development of extraction and clean-up steps for this type of detection might be challenging (Chico et al. Citation2012). The determination of antibiotics in medicated feed, where the drug is added at a known concentration require neither the sensitivity nor the capacity for identification by mass spectrometry, but require accuracy in the analyte quantification. Hence, less resource-consuming and less susceptible to matrix effect techniques, like HPLC with fluorescent and UV detectors are suitable for this purpose (Benito-Peña et al. Citation2009; Gavilán et al. Citation2015; Patyra and Kwiatek Citation2019). Patyra et al. (Citation2013) successfully applied HPLC–DAD techniques for the determination of selected tetracyclines in medicated feed samples (Patyra et al. Citation2013; Patyra and Kwiatek Citation2021). Wang et al. (Citation2008) used supercritical water extraction followed by HPLC with UV detection to quantify oxytetracycline and tetracycline in animal feed. Fiori et al. (Citation2005) performed quality control of doxycycline in medicated premixes by HPLC–DAD.
In this context, the present work aimed to develop and validate a reliable analytical method for the quantification of oxytetracycline (OTC), doxycycline (DC) and chlortetracycline (CTC), in medicated feed samples by HPLC with diode array detection (DAD).
Material and methods
Chemicals, reagents and reference materials
HPLC-grade acetonitrile and hydrochloric acid 37% were purchased from Carlo Erba Reagents Srl (Cornaredo, MI, Italy). HPLC-grade methanol was from VWR International (Radnor, Pennsylvania, USA) and oxalic acid dihydrate from Merck KGaA (Darmstadt, Germany). Ultrapure water was prepared with Milli-Q purification system (Millipore, Bedford, MA, USA). Reference materials (standards) were purchased from Dr.Ehrenstorfer GmbH (Augsburg, Germany) in powder form: chlortetracycline hydrochloride (purity 92.9%), doxycycline hyclate (purity 98%), oxytetracycline hydrochloride (purity 89.62%).
Standard solutions
The standard stock solution containing three target analytes (OTC, CTC, DC) was prepared in methanol at the concentration of 2 mg mL−1, weighting the appropriate amount of each analyte considering the purity declared by the manufacturer. The stock solution was stored at −24 ± 6 °C up to 12 months. Intermediate solutions were prepared by the dilution of the stock solution in methanol at the concentration of 50 µg mL−1 immediately prior to analysis. Working solutions for calibration curve were prepared by the dilution of the intermediate solution at the concentrations 1–2–5–10–20 µg mL−1 in dilution mix acetonitrile:oxalic acid 0.01 M aqueous solution (15:85, v:v).
Sample extraction
Each feed sample was finely ground and homogenised. Based on the declared dosage (dosage reported on the feed label) of the analysed medicated feed, 10 g (declared dosage 40–1000 mg kg−1), 5 g (declared dosage >1000 mg kg−1) or 2 g (declared dosage >2000 mg kg−1) of the ground powder were transferred into a 250 mL plastic bottles. After the addition of 100 mL of extraction solution (1.66% HCl in methanol), bottles were sealed and shaken for 60 min on a horizontal shaker. The mixture was centrifuged at 4000 rpm for 10 min at 10 °C and 5 mL of the supernatant was filtered with the 0.45 µm nylon filters (Laboindustria SpA, Arzergrande, PD, Italy). The filtered extract was properly diluted with dilution buffer acetonitrile:oxalic acid 0.01 M aqueous solution (15:85, v:v) in order to achieve the concentration included in the range of the calibration curve, transferred into the amber vials and analysed within 24 h. Control blank samples along with the fortified samples were subjected to the same extraction and analysis procedure. Blank samples consisted of 5 g of tetracycline-free feed. For fortified sample preparation, 5 g of tetracycline-free feed was spiked at 200 mg kg−1 concentration with 500 µL of tetracyclines stock solution.
Instrumental analysis
The HPLC system was from Agilent (Santa Clara, CA, USA) included degasser, pump Series 1200, autosampler Series 1100, diode array detector Series 1100. The software Agilent ChemStation (Rev. R 04.03) was used for data acquisition and analysis. The chromatographic system was equipped with HPLC column Luna C-18 (length 25 cm, internal diameter 4.6 mm, mean particle size of 5 µm, Phenomenex, Torrance, CA, USA) and HPLC C-18 precolumn, length 4.0 cm internal diameter 3.0 mm and 5 µm mean particle size (Phenomenex, Torrance, CA, USA). The mobile phases consisted of acetonitrile (A) and oxalic acid 0.01 M aqueous solution (B). For each sample, a volume of 10 µL was injected and the chromatographic separation was performed in a 20 min run with the flow rate of 1.0 mL/min. The gradient mode was applied as follows: 0–4 min 10% A and 90% B; 4–8 min 25% A and 75% B; 8–12 min 30% A and 70% B; 13–20 min 10% A and 90% B. The wavelength was set at 360 nm. All the HPLC analyses were performed at room temperature and the system was equilibrated in the working conditions for 30 min prior to batch injection.
For qualitative analysis, the retention time of the analyte was evaluated by overlapping the peaks from the test sample, the fortified sample and the standard solution at the concentration closest to the tetracycline identified in the sample. The UV spectrum of the sample was compared to the UV spectrum of the standard solution at the intermediate concentration by evaluation of the match factor, which is calculated by the software and should result equal to or higher than 950/1000.
The calibration curve was built for each analyte as injected ng of the sample (x-axis) versus the photometric response (y-axis). The calibration curve and the instrument sensitivity were considered acceptable when both linear correlation coefficient r2 was equal or higher than 0.99 and the area of the first point of the calibration curve was significantly quantifiable and corresponded to a signal/noise ratio S/N ≥ 5. In case these requirements were not met, the analysis was repeated. The analyte concentration was calculated through the interpolation on the calibration curve, as ng of the analyte contained in 10 µl of injected extract: analyte concentration (mg kg−1) = (ng injected analyte × dilution factor × 10)/sample weight (g). The same calculation was applied to the fortified sample and the recovery (%) was calculated as the ratio between the measured and the added concentration. The recovery values had to fall within the range of 80–120%, otherwise the analytical session had to be repeated.
Validation procedure
The method validation was performed according to the requirements of the Regulation (EU) 2017/625 Annex III on the characterisation of methods of analysis applied for official controls in food and feed. However, no regulation carrying the specific indications on method validation procedures for medicated feed samples is currently available. Therefore, Regulation (EU) 2021/808 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals was used as a guideline for evaluation of specific method performance criteria (European Commission Citation2021). The following parameters were evaluated: limit of quantification (LOQ), linearity, specificity/selectivity, trueness, repeatability, intra-laboratory reproducibility and uncertainty. The limit of detection (LOD) was not evaluated since the method applies to the samples with declared dosage of target analyte. The applicability of the method for the animal medicated feed was studied in the concentration range 40–1000 mg kg−1 of three target analytes (OTC, CTC and DC) based on the expected concentration in real samples to be analysed. In the present method LOQ is intended as ‘targeted LOQ’ and it is not a calculated value. It was set at 40 mg kg−1 for each target analyte based on the range of concentrations used in medicated feed and evaluated by testing six fortified samples under the conditions of the present method. The linearity was evaluated using standard solution calibration curves with five fortification levels in the range of 1–20 µg mL−1. The selectivity/specificity of the method was tested on six blank samples (cattle, swine, rabbit, poultry and fish feed) to evaluate the absence of interfering responses at the target retention times of oxytetracycline, chlortetracycline and doxycycline. Trueness/recovery (%) was calculated as mean value of each fortification level (40, 200, 500 and 1000 mg kg−1), based on six replicates. The repeatability was assessed as intra-day precision by testing six replicates of three fortification levels in addition to the LOQ concentration (40, 200, 500 and 1000 mg kg−1) prepared on the same day. Standard deviation (SD) and coefficient of variation (CV %) were calculated for each fortification level. For inter-day precision (intra-laboratory reproducibility) evaluation, the same procedure was repeated for three fortification levels (40, 200 and 500 mg kg−1) on three different days. The uncertainty was expressed as maximum expanded relative uncertainty (U(ȳ)) calculated through the bottom-up approach and the following parameters were considered: the uncertainty in the preparation of the calibration curve, in weight measurement and in spiked samples preparation. Studied validation parameters are summarised in .
Table 1. Validation parameters studied for three target analytes (OTC, CTC, DC).
Real samples collection and analysis
Feed samples were collected by competent authorities in the framework of the Italian National Plan on animal feed between January 2021 and December 2023. A total of 103 feed samples were collected from local farms and feed producers. Samples included complete medicated feed (n = 101) and premixes (n = 2) for rabbit (n = 4), swine (n = 93) and aquaculture (n = 6) species. The validated method was applied for samples analysis. Samples were identified as non-compliant if the quantified concentration of the target tetracycline exceeded the tolerance range of 10% applicable to the declared dosage of the medicated feed as per Regulation (EU) 2019/4 Annex IV. In these cases, four replicas of the sample were analysed and the resulting concentration was expressed as the mean value.
Results and discussion
Sample preparation
Animal feed is a complex matrix consisting of a multitude of ingredients of plant and animal origin complemented with various additives. The composition is highly variable based on the animal species the feed is produced for. Therefore, the development of the extraction procedure enabling comparable recoveries from differently composed matrices is necessary. Particularly, the elimination of fat and protein fractions, which may interfere with the analysis, should be performed. Protein precipitation is usually achieved with organic solvents such as acetonitrile and methanol and the extraction may be performed with aqueous solvents containing EDTA to avoid tetracyclines chelation such as McIlvaine–EDTA buffer with different pH (Pérez-Rodríguez et al. Citation2018). Tetracyclines easily bind to the proteins, therefore the addition of strong acids is recommended to help the deproteinization process (Anderson et al. Citation2005). Due to the high solubility of the tetracyclines in methanol, it was used as the extraction solvent in the present method. Methanol was acidified with 1.66% of concentrated hydrochloric acid to achieve optimal overall average recoveries for all the analytes: 108% (SD 4.4) for oxytetracycline, 106% (SD 4.5) for chlortetracycline and 106% (SD 4.0) for doxycycline. The use of diluted hydrochloric acid did not ensure the same extraction performance.
For trace level analytes in feed (e.g. carry-over analysis) more sophisticated extraction and clean-up protocols are applied to achieve appropriate recoveries and eliminate interferences. In the medicated feed, the concentrations are usually in the mg kg−1 range and rapid, simple extraction is properly performed without additional clean-up and concentration steps. Instead, the dilution step helps to lower the matrix interferences.
Chromatographic analysis
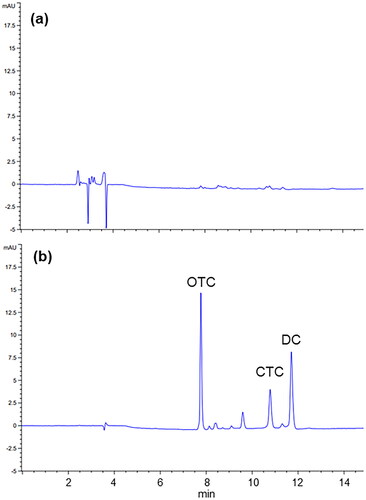
C18 columns are commonly used for tetracyclines separation (Anderson et al. Citation2005). In the present method, the Phenomenex Luna C18 column was used and coupled to C18 guard column for analytical column protection. Acetonitrile and 0.01 M oxalic acid were used as mobile phases. Acidic solutions enhance the detection of tetracyclines in the 350–365 nm range and, particularly, the use of low-concentrated oxalic acid has been frequently reported for the improvement of peak shape and tailing avoidance (Anderson et al. Citation2005). The applied gradient allowed the optimal separation of OTC, CTC and DC in a 20 min long chromatographic run ().
Method validation
The applicability range from 40 to 1000 mg kg−1 for medicated animal feed matrices was chosen based on the expected concentrations utilised for standard medicated feed for different animal species.
To verify the absence of significant instrumental response at the retention times of three target tetracyclines, six blank samples were treated and analysed under the method conditions and no interferences were identified.
The calibration curve was built as measured concentration vs. the instrumental response (area of the chromatographic peak) on five fortification levels (1–2–5–10–20 µg mL−1). Three replicates were analysed for each concentration level. The least squares regression line was built and the obtained r2 resulted to be higher than 0.99.
The trueness of the method was assessed through the recovery percentage at four concentration levels within the validation range, including the LOQ level (). The values obtained for the recovery fell within the range between 80% and 120% and were considered acceptable. The repeatability of the method was assessed as the intra-day precision and the calculated values for standard deviations and coefficients of variation are reported in the .
Table 2. Validation parameters studied for trueness, repeatability and intra-laboratory reproducibility calculations for three target analytes (OTC, CTC, DC).
For inter-day precision (intra-laboratory reproducibility) evaluation, 18 fortified samples were analysed in three analytical sessions, performed on three different days. shows the results obtained for each molecule. Since no specific method performance criteria are available for feed samples, to evaluate the acceptability of the CV values, the reference value of 16% reported in the Regulation (EU) 2021/808 was taken as maximum acceptable point and the method fulfilled the requirement (European Commission Citation2021).
The uncertainty was expressed as maximum extended relative uncertainty (U(ȳ)), the effective degrees of freedom (ν), calculated using the Welch–Satterthwaite formula, the calculated coverage factor (k) and a confidence level (p) of 95% were considered and the results are presented in .
Table 3. Evaluated uncertainty parameters.
The maximum extended relative uncertainty was used for each quantified sample to calculate the measurement uncertainty by applying the following equation, where C is the obtained concentration of the analyte:
(1)
(1)
Application to real samples analysis
The described method was applied for quantification of oxytetracycline, doxycycline and chlortetracycline in medicated feed for livestock. A total of 103 samples were analysed during the years 2021–2023 in the framework of the National Plan for animal feed. The compliance with the declared antibiotic dosage in the samples was assessed. Quantified concentration of eight swine and one fish feed samples were found to be non-compliant compared to the declared dosages of target tetracycline (). In all cases, the quantified concentrations were lower than the content declared on the label. In one sample the declared dosage of chlortetracycline (2000 mg kg−1) was almost 20-fold higher than the quantified concentration resulting in 114 mg kg−1.
Table 4. Non-compliant tetracycline concentrations in analysed feed samples.
Moreover, in one analysed swine feed sample the reported dosage of chlortetracycline on the label was found compliant, however an undisclosed presence of doxycycline, not declared on the label, was identified at the concentration of 57 mg kg−1 (). In this case, most likely the cross-contamination occurred along the production chain. The sample would exceed 50 fold the maximum residue limit of 1 mg kg−1 established by national legislation according to the requirements of the Regulation (EU) 2019/4. The overall non-compliance rate resulted in 8.7%.
Conclusions
The present work reported the validation of an easy and fast analytical method for quantification of three tetracyclines (oxytetracycline, chlortetracycline and doxycycline) in medicated feed samples applicable to different animal species. Validation was carried out according to Regulation (EU) 2017/625 Annex III and where appropriate, method performance parameters listed in Regulation (EU) 2021/808 were applied as guideline, despite the fact that the dosage evaluation in feed samples is not within the scope of the present regulation. The validated method is based on the HPLC–DAD detection following a simple extraction procedure with acidified methanol. Each analyte is quantified through the interpolation on the calibration curve prepared for each analytical session. The analytical method was applied for the analysis of 103 feed samples, and in eight swine and one fish medicated feed samples non-compliant tetracycline concentrations were identified. The incorrect antibiotic dosages in medicated feed are potential threats for animal health, where the underdosing of the antibiotics caused by overestimation of the in-feed concentration declared on the label may result in ineffective treatments, or on the other hand, the underestimation of the declared dosage may lead to adverse effects. Potential consequences for human health, such as the presence of antibiotic residues in food derived from incorrectly treated animals are also an important concern and therefore, the compliance of the antibiotic’s dosage reported on the label of the medicated feed should be accurately monitored by competent authorities. Given the necessity for precise quantification to ensure compliance with declared dosage in medicated feed, the intricate nature of the feed matrix, and the susceptibility of tetracyclines to matrix effects in LC–MS/MS, when identification is not the primary focus, LC–UV detection emerges as a viable solution. The reported method is a useful tool for monitoring programmes aiming for the determination of correct dosage of tetracyclines in medicated feed and is suitable for application in routine laboratories without need of sophisticated equipment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, EB, upon reasonable request.
References
- Anderson CR, Rupp HS, Wu WH. 2005. Complexities in tetracycline analysis – chemistry, matrix extraction, cleanup, and liquid chromatography. J Chromatogr A. 1075(1–2):23–32. doi: 10.1016/j.chroma.2005.04.013.
- Apley MD, Bush EJ, Morrison RB, Singer RS, Snelson H. 2012. Use estimates of in-feed antimicrobials in swine production in the United States. Foodborne Pathog Dis. 9(3):272–279. doi: 10.1089/fpd.2011.0983.
- Benito-Peña E, Urraca JL, Moreno-Bondi MC. 2009. Quantitative determination of penicillin V and amoxicillin in feed samples by pressurised liquid extraction and liquid chromatography with ultraviolet detection. J Pharm Biomed Anal. 49(2):289–294. doi: 10.1016/j.jpba.2008.11.016.
- Brown K, Uwiera RRE, Kalmokoff ML, Brooks SPJ, Inglis GD. 2017. Antimicrobial growth promoter use in livestock: a requirement to understand their modes of action to develop effective alternatives. Int J Antimicrob Agents. 49(1):12–24. doi: 10.1016/j.ijantimicag.2016.08.006.
- Chico J, van Holthoon F, Zuidema T. 2012. Ion suppression study for tetracyclines in feed. Chromatogr Res Int. 2012:1–9. doi: 10.1155/2012/135854.
- Desmarchelier A, Anizan S, Minh Tien M, Savoy MC, Bion C. 2018. Determination of five tetracyclines and their epimers by LC–MS/MS based on a liquid–liquid extraction with low temperature partitioning. Food Addit Contam – Part A. 35(4):686–694.
- Dewell GA, Rademacher CJ, Sato Y. 2022. Review of regulations and indications for the use of in-feed antimicrobials for production animals. J Am Vet Med Assoc. 260(S3):S129–S132. doi: 10.2460/javma.22.07.0300.
- European Commission. 2010. Commission Regulation (EU) No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union. L15:1–72.
- European Commission. 2017. Regulation (EU) 2017/625 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products. Off J Eur Union. 95(1):1–142.
- European Commission. 2021. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to. Off J Eur Union. 180(401):84–109.
- [EMA] European Medicine Agency. 2019. Categorisation of antibiotics in the European Union; Answer to the request from the European Commission for updating the scientific advice on the impact on public health and animal health of the use of antibiotics in animals [accessed 2023 Dec 20]. https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific-advice-impact-public-health-and-animal-health-use-antibiotics-animals_en.pdf.
- European Parliament and Council. 2019. Regulation (EU) 2019/4 of the European Parliament and of the Council of 11 December 2018 on the manufacture, placing on the market and use of medicated feed, amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and repealing. Off J Eur Union. 4(178):1–23.
- Fiori J, Grassigli G, Filippi P, Gotti R, Cavrini V. 2005. HPLC-–DAD and LC–ESI–MS analysis of doxycycline and related impurities in doxipan mix, a medicated premix for incorporation in medicated feedstuff. J Pharm Biomed Anal. 37(5):979–985. doi: 10.1016/j.jpba.2004.06.017.
- Gavilán R, Nebot C, Miranda J, Martín-Gómez Y, Vázquez-Belda B, Franco C, Cepeda A. 2015. Analysis of tetracyclines in medicated feed for food animal production by HPLC–MS/MS. Antibiotics. 5(1):1. doi: 10.3390/antibiotics5010001.
- Georgaki D, Vandael F, Cardoso de Carvalho Ferreira H, Filippitzi ME, De Backer P, Devreese M, Dewulf J, Croubels S. 2023. Qualitative risk assessment of homogeneity, stability, and residual concentrations of antimicrobials in medicated feed and drinking water in pig rearing. BMC Vet Res. 19(1):9. doi: 10.1186/s12917-022-03555-3.
- Ghimpețeanu OM, Pogurschi EN, Popa DC, Dragomir N, Drăgotoiu T, Mihai OD, Petcu CD. 2022. Antibiotic use in livestock and residues in food—a public health treat: a review. Foods. 11(10):1430. doi: 10.3390/foods11101430.
- Granados-Chinchilla F, Rodríguez C. 2017. Tetracyclines in food and feedingstuffs: from regulation to analytical methods, bacterial resistance, and environmental and health implications. J Anal Methods Chem. 2017:1315497. doi: 10.1155/2017/1315497.
- Jank L, Martins MT, Arsand JB, Ferrão MF, Hoff RB, Barreto F, Pizzolato TM. 2018. An LC–ESI–MS/MS method for residues of fluoroquinolones, sulfonamides, tetracyclines and trimethoprim in feedingstuffs: validation and surveillance. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 35(10):1975–1989. doi: 10.1080/19440049.2018.1508895.
- Kahn LH. 2017. Antimicrobial resistance: a one health perspective. Trans R Soc Trop Med Hyg. 111(6):255–260. doi: 10.1093/trstmh/trx050.
- Koutsoumanis K, Allende A, Alvarez-Ordóñez A, Bolton D, Bover-Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F. 2021. Maximum levels of cross-contamination for 24 antimicrobial active substances in non-target feed. Part 12: tetracyclines: tetracycline, chlortetracycline, oxytetracycline, and doxycycline. EFSA J. 19(10) e06852.
- Mehdi Y, Létourneau-Montminy MP, Gaucher ML, Chorfi Y, Suresh G, Rouissi T, Brar SK, Côté C, Ramirez AA, Godbout S. 2018. Use of antibiotics in broiler production: global impacts and alternatives. Anim Nutr. 4(2):170–178. doi: 10.1016/j.aninu.2018.03.002.
- Ministero della Salute. 2017-2024. Elenco dei Medicinali veterinari autorizzati alla commercializzazione o in stato di sospensione. [List of authorized and suspended veterinary medicines]. Italian. [accessed 2023 Dec 20]. https://www.dati.salute.gov.it/dati/dettaglioDataset.jsp?menu=dati&idPag=90.
- Ministero della Salute. 2021. Piano nazionale di controllo ufficiale sull’alimentazione animale. [National plan for official controls on animal feed]. Italian. [accessed 2024 Mar 26]. https://www.salute.gov.it/imgs/C_17_pubblicazioni_2997_allegato.pdf.
- Patyra E, Kowalczyk E, Kwiatek K. 2013. Development and validation method for the determination of selected tetracyclines in animal medicated feedingstuffs with the use of micellar liquid chromatography. Anal Bioanal Chem. 405(21):6799–6806. doi: 10.1007/s00216-013-7117-5.
- Patyra E, Kwiatek K. 2017. Development and validation of multi-residue analysis for tetracycline antibiotics in feed by high performance liquid chromatography coupled to mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 34(9):1553–1561. doi: 10.1080/19440049.2017.1364430.
- Patyra E, Kwiatek K. 2019. HPLC–DAD analysis of florfenicol and thiamphenicol in medicated feedingstuffs. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 36(8):1184–1190. doi: 10.1080/19440049.2019.1619943.
- Patyra E, Kwiatek K. 2021. Comparison of HPLC–DAD and LC–MS techniques for the determination of tetracyclines in medicated feeds using one extraction protocol. Chromatographia. 84(8):741–749. doi: 10.1007/s10337-021-04058-3.
- Pérez-Rodríguez M, Pellerano RG, Pezza L, Pezza HR. 2018. An overview of the main foodstuff sample preparation technologies for tetracycline residue determination. Talanta. 182(January):1–21. doi: 10.1016/j.talanta.2018.01.058.
- Santos-Santórum Suárez C, Sanders P, Gaugain M, Viel A, Paboeuf F, Taillandier JF, Houée P, Valentin C, Perrin-Guyomard A. 2023. Selection of antibiotic resistance in pigs after exposure to feed cross-contaminated with oxytetracycline. Vet Microbiol. 287(June):109924. doi: 10.1016/j.vetmic.2023.109924.
- Stolker AAM, Manti V, Zuidema T, van Egmond H, Deckers ER, Herbes R, Hooglugt J, Olde Heuvel E, de Jong J. 2013. Carry-over of veterinary drugs from medicated to non-medicated feeds in commercial feed manufacturing plants. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 30(6):1100–1107. doi: 10.1080/19440049.2013.794308.
- Vandael F, Filippitzi ME, Dewulf J, Daeseleire E, Eeckhout M, Devreese M, Croubels S. 2019. Oral group medication in pig production: characterising medicated feed and drinking water systems. Vet Rec. 185(13):405–405. doi: 10.1136/vr.105495.
- Wang L, Yang H, Zhang C, Mo Y, Lu X. 2008. Determination of oxytetracycline, tetracycline and chloramphenicol antibiotics in animal feeds using subcritical water extraction and high performance liquid chromatography. Anal Chim Acta. 619(1):54–58. doi: 10.1016/j.aca.2008.01.026.