ABSTRACT
Lignocellulolytic enzymes play an important role in various industrial applications as well as the sustainable valorisation of lignocellulosic materials. Enzyme production using lignocellulosic fungi has shown great advantages such as high enzyme diversity, high production efficiency, and the availability of solid waste as raw materials. Agricultural waste, an abundant and non-food competitive feedstock, can be used to produce fungal lignocellulolytic enzymes. Pretreatment helps break down the complex structure of the raw material, thereby significantly improving product yield but also requiring more energy consumption. Multiple fermentation technologies, including submerged fermentation, solid-state fermentation, and co-culture, can be used for producing lignocellulolytic enzymes. Process optimisation may promote the yield and productivity of such enzymes without additional investment. Genetic engineering is also useful for enhancing enzyme production to meet industrial requirements. This review summarises the research progress in the fungal production of lignocellulolytic enzymes from various agricultural wastes via advanced fermentation strategies. It aims to provide technical references for the scale-up production of fungal lignocellulolytic enzymes.
1. Introduction
The global enzyme market size has been growing and is expected to reach $8.7 billion by 2026 (Dewan Citation2021) owing to increasing demands for adapting environmental-friendly solutions and finding sustainable alternatives to fossil fuels. Lignocellulosic enzymes account for more than 20% of enzyme sales in the global market (Leite et al. Citation2021). Combined with specific techniques, these enzymes have shown significant potential in many industrial and environmental applications, such as paper making, food processing, and pollutant degradation (Chukwuma et al. Citation2020; Saldarriaga-Hernández et al. Citation2020; Saini and Sharma Citation2021). Furthermore, lignocellulolytic enzymes can depolymerise lignocellulose into fermentable sugars under mild conditions. As a type of microbial enzyme, they are also highly resistant to the inhibitory effects of other microbial metabolites (Hosseini Koupaie et al. Citation2019). These properties have aroused enormous research interest in utilising lignocellulolytic enzymes to facilitate the bioconversion of lignocellulosic materials into value-added products, such as biofuels (Raud et al. Citation2019) and biopolymers (Wang et al. Citation2021; Wang et al. Citation2023). On the other hand, lignocellulose is a natural and ideal inducer for the production of lignocellulolytic enzymes. Agricultural residues are the representative form of lignocellulosic biomass waste. Large amounts of agricultural waste have been generated due to the gradually expanded agricultural production with population growth, resulting in negative environmental impacts. Utilising them as feedstocks to produce lignocellulosic enzymes is a promising approach to valorising these wastes while easing the environmental pressure of waste disposal.
Lignocellulolytic enzymes can be produced extracellularly by both bacteria and fungi. Compared with lignocellulolytic bacteria, the lignocellulolytic fungus can produce lignocellulolytic enzymes with higher diversity due to their more powerful metabolic systems for extracellular enzyme synthesis (Andlar et al. Citation2018). They also have higher adaptability to low-moisture environments to produce lignocellulolytic enzymes from solid wastes with minor pretreatment. It is conducive to improving enzyme production efficiency and reduces the risk of contamination and enzyme degradation (Leite et al. Citation2021). Lignocellulolytic fungi are grouped based on their different effects and preferential degradation to lignocellulosic polymers. White-rot fungi show excellent efficiency in the degradation of lignin. Soft-rot fungi have the highest cellulolytic and hemicellulolytic enzyme system, followed by white-rot fungi and brown-rot fungi (Sista Kameshwar and Qin Citation2018). lists the enzyme-producing fungi, enzyme characteristics, and recent applications of various lignocellulolytic enzymes. However, their widespread industrial applications have been facing the major challenge of insufficient enzyme productivity and selectivity. Therefore, comprehensive knowledge is still needed to improve production efficiency by optimising fermentation conditions, simplifying processes, and exploring new technologies.
Table 1. Recent applications of various lignocellulolytic enzymes from different wild-type fungal cultures.
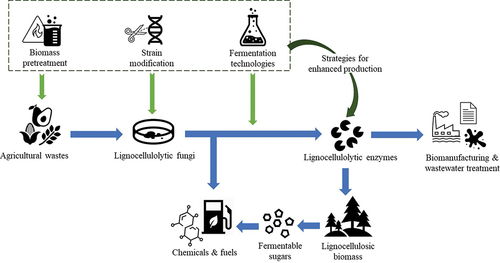
This review discussed the recent studies on producing fungi-derived lignocellulolytic enzymes from various agricultural wastes. The pretreatment of the raw materials was also included. In addition, the significant enhancements in enzyme production by applying advanced fermentation technologies and strain modifications were addressed.
2. Lignocellulolytic enzymes
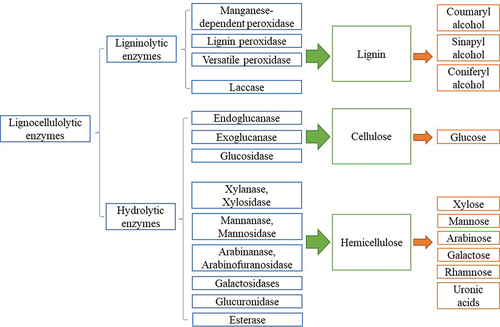
Lignocellulose mainly contains 5%–30% lignin, 20%–35% hemicellulose, and 35%–50% cellulose. The proportion of major components and the composition of their monomers may vary significantly in different plants. Therefore, the complete depolymerisation of lignocellulosic materials relies on the synergistic action of various lignocellulolytic enzymes. Based on the target substrate, these enzymes can be divided into ligninolytic enzymes and hydrolytic enzymes. The ligninolytic enzymes work on the disruption of lignin, whereas the hydrolytic enzymes are responsible for the hydrolysis of carbohydrate polymers. shows an overview of lignocellulolytic enzymes involved in the degradation of lignocellulose.
2.1. Ligninolytic enzymes
Lignin is an amorphous phenolic polymer with a complex crosslinked structure composed of phenylpropane derivatives. Lignin in different plants contains different percentages of monomers, linked to each other mainly by carbon-carbon bonds or ester bonds (Ralph et al. Citation2019). Lignin degradation is an oxidative process involving two groups of enzymes: peroxidase and laccase. Peroxidase requires hydrogen peroxide as the electron acceptor to initiate the catalysing of non-phenolic compounds. Laccase uses oxygen as the oxidant to oxidise both phenolic and non-phenolic compounds (Chio et al. Citation2019).
2.2. Hydrolytic enzymes
Cellulose and hemicellulose are the two major carbohydrate polymers in lignocellulose. Cellulose is a linear polysaccharide consisting of hundreds or thousands of glucose molecules linked by β-1,4-glycosidic bonds (McNamara et al. Citation2015). In a typical cellulase system, endoglucanases act on the amorphous regions of cellulose and cleave glycosidic bonds internally, while exoglucanase act on the crystalline regions of cellulose to release β-cellobiose. The generated oligomers and cellobiose are then hydrolysed to glucose by glucosidase (Barbosa et al. Citation2020). Hemicellulose is a heterogeneous polymer composed of different five- and six-carbon sugars linked by β-1,3- and β-1,4-glycosidic bonds, and its branch chains may be characterised by uronic acids. In addition, hemicellulose may be closely bound to lignin and cellulose by aromatic ester and hydrogen bonds, respectively (Ge et al. Citation2018; Rao et al. Citation2023). Xylanases hydrolyse xylan and produce xylo-oligomers, which are hydrolysed by xylosidases to release xylose. Similarly, mannanases and mannosidases act on mannan together to release mannose. Other accessorial enzymes also play an important role. Arabinofuranosidase hydrolyzes the covalent bonds between arabinose and xylose. Esterases cleave the ester bonds uronic acids and oligomers (Houfani et al. Citation2020).
3. Fungal production of lignocellulolytic enzymes from agricultural wastes
3.1. Food crop waste
When food crops are harvested and processed, unwanted parts, such as stems, leaves, and husks, are discarded as wastes. Currently, wheat bran is one of the main substrates for the fungal production of various lignocellulolytic enzymes. By cultivating Myceliophthora thermophila (M. thermophila) with wheat bran, da Rosa-Garzon NG et al. (Citation2022) reported the production of cellulases (309.8 U/g, Day 1), xylanase (4,105 U/g, Day 1) and glucosidase (178.4 U/g, Day 2). Garcia et al. (Citation2015) obtained high production of glucosidase (274 U/g) from wheat bran by Lichtheimia ramose. The produced enzyme remained stable at a wide range of pH (3.5–10.5) and highly active under up to 10% ethanol. Cotylidia pannosa can be cultivated on wheat bran to produce an efficient lignocellulolytic enzyme cocktail containing cellulase (20 U/mL), xylanase (17 U/mL), and laccase (13 U/mL) (Sharma et al. Citation2016). Besides, rice waste biomass was identified as an ideal substrate for Streptomyces sp. to produce endoglucanase (132.6 U/gds), exoglucanase (14.6 U/gds), cellobiase (125.6 U/gds), and xylanase (342.5 U/gds). The enzyme complex had good resistance to high pH (5–8), high temperature (50–80 °C), and organic solvents (Saratale et al. Citation2017). Rice husk also showed good potential in laccase production (6.9 U/mL) by Trametes versicolor (T. versicolor) (Perdani et al. Citation2020). Auricularia auricula (A. auricula) can utilise alkali-treated corn stalk to generate glucosidase (2.92 IU/g, Day 6) and exoglucanase (2.92 IU/g, Day 8) (Lu et al. Citation2022). Astolfi et al. (Citation2019) compared the cellulolytic enzyme production by Trichoderma reesei (T. reesei) among four different wastes, and soybean hulls were found to provide maximum activity xylanase (1,130.70 U/g, Day 7), in addition to endoglucanase (6.72 U/g, Day 15) and exoglucanase (5.45 U/g, Day 3). Ozcirak Ergun and Ozturk Urek (Citation2017) investigated the ligninolytic enzyme production from potato peel waste by Pleurotus ostreatus (P. ostreatus). The highest laccase activity (6,708.3 U/L, Day 17) was achieved with dry peels, while the highest MnP activity (2,503.6 U/L, Day 17) was determined in neutralised fresh peels. Martarello et al. (Citation2019) obtained galactosidase with the activity of 24.64 U/mL after culturing Aspergillus niger (A. niger) on soybean residues for 7 days.
3.2. Cash crop waste
Cash crops, also known as “technical crops”, generally refer to crops with relatively high value as industrial raw materials. In the palm oil industry, a vast amount of oil palm residues is generated in oil palm plantations and oil palm refineries. Taking oil palm fruit bunches as the substrates, Aspergillus sp. produced xylanase (54.32 U/g, Day 8), lignin peroxidase (13.41 U/g, Day 8) but low activity cellulase (Orozco Colonia et al. Citation2019). Ezeilo et al. (Citation2020) cultivated Trichoderma asperellum (T. asperellum) and Rhizopus oryzae (R. oryzae) on oil palm leaves. T. asperellum generated endoglucanase (59.64 U/g, Day 4), exoglucanase (9.58 U/g, Day 4), glucosidase (118.1 U/g, Day 4) and xylanase (175.91 U/g, Day 5). R. oryzae generated the same enzyme cocktail but all at lower levels and required a longer cultivation period to reach maximum activity, endoglucanase (41.62 U/g, Day 6), exoglucanase (9.58 U/g, Day 5), glucosidase (113.07 U/g, Day 5) and xylanase (162.68 U/g, Day 6). Oil palm decanter cake after the refining process can also be used by Pseudolagarobasidium sp. to yield laccase (5.841 U/gds, Day 7) and manganese peroxidase (5.156 U/gds, Day 7) (Thamvithayakorn et al. Citation2019). Sugarcane bagasse, the main by-product of sugarcane sugar production, is a sustainable biomass resource rich in both sugar and fibre. Namnuch et al. (Citation2021) confirmed that sugarcane bagasse was a good source for producing endoglucanase (1.27 U/mL), cellulase (0.72 U/mL), and xylanase (376.81 U/mL), after 14 days of fermentation by Aspergillus flavus (A. flavus). Another strain, A. niger, was evaluated on sugarcane bagasse and brewery spent grain, showing the highest activity for cellulase (6.23 U/gds, Day 5) and xylanase (1,400.80 U/gds, Day 5) on brewery spent grain and sugarcane bagasse, respectively (Moran-Aguilar et al. Citation2021). Ravindran et al. (Citation2019) used spent coffee waste as the sole carbon source for xylanase production by A. niger, with the activity of 4,649 IU/gds on Day 5. Favaro et al. (Citation2020) identified the best ratio of coffee waste and wheat bran as 1:1 for the highest production of mannanase (45 IU/g, Day 5) by A. niger. By combining olive pomace with winery waste, Filipe et al. (Citation2020) made it possible to achieve the maximum production of xylanase (189.1 U/g, Day 7), cellulase (56.3 U/g, Day 4) and glucosidase production (10.9 U/g, Day 2) by Aspergillus ibericus.
3.3. Fruit waste
Fruit wastes, including stems, leaves, peel or shell, seeds, and bagasse, usually contain a higher number of fermentable sugars than crop wastes. Backes et al. (Citation2022) studied the laccase production from pineapple crowns by T. versicolor and obtained a maximal laccase activity of 60.73 U/g in 7-day cultures. After 21-day cultivation of Trametes hirsute on pineapple leaf waste, extracellular laccase enzyme production reached a maximum activity of 3,003.2 U/mL (Chablé-Villacis et al. Citation2021). Gooruee et al. (Citation2022) examined different species of Trichoderma fungi for a 2-day enzyme production from lemon peels. Trichoderma afroharzianum showed the highest activity of exoglucanase (5.42 U/mL), endoglucanase (6.02 U/mL), total cellulase (10.96 U/mL), and xylanase (4.11 U/mL), while Trichoderma lixii showed the highest activity of glucosidase (10.17 U/mL). Akpinar and Ozturk Urek (Citation2022) directly applied peach waste for the lignocellulolytic enzyme production by Pleurotus eryngii (P. eryngii), namely manganese peroxidase (476.04 U/L), lignin peroxidase (895.80 U/L), endoglucanase (11.55 U/mL), xylanase (3.27 U/mL), exoglucanase (4.42 U/L), glucosidase (34.77 U/L). A similar enzyme cocktail was successfully produced by the same P. eryngii strain using cherry waste (Akpinar and Ozturk Urek Citation2020). A. niger was able to grow on melon residues and synthesise endoglucanase (1.21 U/mL, Day 2), xylanase (11.00 U/mL, Day 8), and laccase (18.23 U/mL, Day 5) (Rodríguez-Luna et al. Citation2022). When subject to a 3-day fermentation on passion fruit peel, A. niger produced exoglucanase (1.243 U/mL) and endoglucanase (1.714 U/mL) (Silva et al. Citation2019). de Oliveira Júnior SD et al. (Citation2022) reused guarana processing residues to produce endoglucanase (0.84 U/g, Day 8), xylanase (1.00 U/g, Day 7), laccase (176.23 U/mL, Day 5) in addition to phenolic compounds. de Almeida Antunes Ferraz JL et al. (Citation2018) fermented yellow mombin residue using Penicillium roqueforti, resulting in a crude enzyme extract rich in xylanase (14 IU/g).
3.4. Forestry waste
Forestry wastes are generated from forest harvesting and regenerating, for example, wood chips, bark, sawdust, timber slash, and mill scrap. They are lignocellulosic wastes with a relatively high content of lignin. Sunardi et al. (Citation2016) studied the lignocellulolytic enzyme activity during the degradation of Picea jezoensis (spruce) wood by Porodaedalea pini. Ligninolytic enzymes generally had low activity, laccase (0.044 nkat/mg, Day 30), lignin peroxidase (0.368 nkat/mg, Day 120), and manganese peroxidase (2.100 nkat/mg, Day 120). Xylanase (89.788 nkat/mg), endoglucanase (70.425 nkat/mg), and exoglucanase (15.559 nkat/mg) activities reached their maximum levels on Day 60. Laccase activity from P. ostreatus and Flammulina velutipes (F. velutipes) was investigated with Populus beijingensis (hybrid poplar). Maximum laccase activity for both strains was observed on Day 7, except that P. ostreatus had a much higher activity (303.52 U/L) than F. velutipes (40.62 U/L) (An et al. Citation2021). Among the single cultivations of Gloeophyllum sepiarium (G. sepiarium) on birch, spruce, and pine wood, pine wood as the sole carbon source gave the most active endoglucanase (2.2 µkat/L, Day 24) and xylanase (6.2 µkat/L, Day 24). The highest activities of glucosidase (~0.06 µkat/L, Day 24), mannosidase (~0.018 µkat/L, Day 24), and xylosidase (~0.015 µkat/L, Day 24) were detected on birch, while the highest activity of galactosidase (0.18 µkat/L, Day 35) was on spruce. The ligninolytic enzyme activities were almost flat in all hardwood and softwood cultivations (Sugano et al. Citation2021). The research of Vázquez-Montoya et al. (Citation2020) demonstrated that Penicillium funiculosum displayed the highest activities of endoglucanase (1,683 U/L, Day 4), exoglucanase (1,700 U/L, Day 4) and glucosidase (924 U/L, Day 6) on Moringa oleifera (benzolive tree) straw. Qi et al. (Citation2022) compared the biodegradation abilities of serval fungi species in Dendrocalamus sinicus (bamboo) sawdust. The white-rot fungus T. versicolor produced the full spectrum of lignocellulolytic enzymes, exoglucanase (40.12 U/mL), endoglucanase (~10 U/mL), hemicellulose (~25 U/mL), laccase (~440 U/mL), manganese peroxidase (500 U/mL), and lignin peroxidase (~330 U/mL). The brown-rot fungi Gloeophyllum trabeum and Rhodonia placenta had higher hydrolytic enzyme activities, exoglucanase (55.18 and 180.26 U/mL), endoglucanase (~130 and 180.26 U/mL), hemicellulose (186.6 and 169.65 U/mL), but much lower ligninolytic enzyme activities, laccase (<3 U/mL), (<5 U/mL), lignin peroxidase (~58 and ~33 U/mL).
3.5. Pretreatment of agricultural waste
One of the major challenges in producing lignocellulolytic enzymes from agricultural wastes is the highly complex and recalcitrant structure of lignocellulose. Typically, biomass is pretreated to disintegrate the lignin from cellulose and hemicellulose, allowing the microorganism to access the sugars present within the holocellulose fraction. In addition, agricultural residue is a mixed waste of non-uniform sizes and compositions, which may contain many factors that inhibit the growth of fungi. Altering these inhibitors by pretreatment has significant impacts on fungal cultivation. The common pretreatment approaches are classified into physical, chemical, and physicochemical. Physical pretreatment techniques, including mechanical, ultrasonic, microwave, and thermal pretreatment, are used to reduce the particle sizes and promote the efficiency of subsequent treatments. Chemical pretreatment applies acids, alkalis, oxidation, or organic solvent to disrupt the lignocellulose structure and enhance cellulose digestibility. Acid is commonly used to dissolve hemicellulose and convert the dissolved hemicellulose into fermentable sugars. Concentrate acid forms inhibitors and causes reactor corrosion. Thus, dilute acid is preferred, with a rapid reaction rate favouring continuous biomass processing. Alkaline is another available reagent for biomass pretreatment. It can remove lignin and promote deacetylation and uronic acid removal from hemicellulose. In addition, the mixed reagents can improve the performance of pretreatment. For example, green liquid, a mixture of sodium carbonate and sodium sulphide, increased the conversion rate by reducing cellulose crystallinity and polymerisation degree (Malik et al. Citation2022). To further improve the biodegradability of agricultural wastes, physicochemical pretreatment methods combine the advantages of physical and chemical treatments, such as steam explosion, alkali-heat pretreatment, and ammonia fibre expansion (Awogbemi and Von Citation2022). Lu et al. (Citation2022) compared the cultivations of A. auricula on corn stalks pretreated with alkali, alkali-ozone, ozone, and high-temperature water (control). The alkali pretreatment group contributed to the maximum growth of mycelium (1.2 times of control) and the highest activity of glucosidase (3.4 times), whereas the ozone treatment group showed the highest activity of laccase (2.1 times). Perdani et al. (Citation2020) proposed that the laccase yields by T. versicolor from cornstalk, rice husk, and bagasse after steam explosion were 1.61, 1.86, and 1.66 times as high as those without pretreatment. The production of cellulase by A. niger using brewery spent grain autoclaved at 130°C for two hours was 1.8 times that of untreated (Moran-Aguilar et al. Citation2021). However, the additional energy consumption and waste pollutants caused by the pretreatment process cannot be ignored. Blindly pretreating raw materials for optimal enzyme production at all costs is impractical and unsustainable.
4. Fermentation technologies for enzyme production
The purpose of developing a fermentation process is to increase productivity by adjusting fermentation conditions without adding additional processes, thereby reducing production costs, and maximising resource utilisation. Application of appropriate fermentation strategies can facilitate the fungal transformation of agricultural wastes, leading to higher yields of target lignocellulolytic enzymes.
4.1. Submerge fermentation (SmF) and Solid-State fermentation (SSF)
SmF and SSF are the most used fermentation technologies for lignocellulolytic enzyme production by microbes. SmF is a process where microorganism grows in the liquid broth, while SSF takes place in the absence or near absence of free water. Since SmF is technically easier to implement with process controls, it has been more readily available for industrial production. The better control of fermentation parameters and nutrient composition in SmF contribute to a high stability of enzyme production. On the other hand, SSF offers several advantages for those fungi species that struggle in liquid environments. The risk of contamination is substantially reduced in SSF due to the low moisture content. For fermentations with solid agricultural waste, using SSF may simplify the pretreatment of feedstocks, resulting in reduced energy consumption and cost savings. However, for any enzyme production of interest, there is no magic recipe that works for all strains. Liu et al. (Citation2020) investigated the expression of lignocellulolytic enzymes from corn stover powder by Phanerochaete chrysosporium (P. chrysosporium). Most enzyme activities from SSF were 2 to 2.3 times as high as those from SmF, except xylanase activity from SSF was 20% lower. Both enzyme cocktails showed similar hydrolysis results at high substrate loading, but the weaker enzyme cocktail from SmF was more effective at low substrate loading due to the higher ratio of carbohydrate-binding enzymes in SmF. Elegbede and Lateef (Citation2018) selected eight fungi strains to produce xylanases from corncob in SmF and SSF. SSF yielded higher xylanase activity (1.1 to 3.4 times that of SmF) in half of the strains, Aspergillus fumigatus, Botryodiplodia sp., A. flavus, A. niger. In the other half, up to 80% of xylanase activity was lost in SSF. Enzyme productions by P. ostreatus were compared in SSF and SmF using grape pomace as the substrate. The highest endoglucanase activity of 0.93 U/g was obtained in SmF (0.07 U/g in SSF), whereas the maximum laccase activity of 26,247 U/g was observed in SSF (4,447 U/g in SmF) (Papadaki et al. Citation2019). In sequential SSF-SmF of palm empty fruit bunches by Aspergillus tubingensis (A. tubingensis), activities of cellulase and xylanase (89.6 U/g and 196.8 U/g) were significantly higher than in SSF alone (40.09 U/g and 62.43 U/g) or SmF alone (66.02 U/g and 158.5 U/g) (Intasit et al. Citation2021).
4.2. Co-cultivation
In nature, the degradation of lignocellulosic materials is a long process accomplished by a variety of organisms with different specialities in degrading different portions of lignocellulose. Co-cultivation is a technique of growing two or more fungi with complementary enzyme activities to create a more diverse and robust enzyme cocktail, mimicking the natural process of biodegradation. This approach holds great promise for industrial applications, but targeted research is still needed on strain interactions, synergistic mechanisms, and strain pairing. Sugano et al. (Citation2021) found that combining the white-rot fungus, Bjerkandera adusta, with one brown-rot fungus, G. sepiarium or Antrodia sinuosa, in co-cultivations displayed synergistic glucosidase and galactosidase activities. Co-cultivation of T. reesei and Monascus purpureus upregulated the expression of hydrolytic enzymes compared with their single cultivation. The obtained crude enzymes exhibited significant efficiency in hydrolysing wheat straw (Fatma et al. Citation2021). Ming et al. (Citation2019) selected 31 strains to co-culture on distiller’s grain, among which A. niger co-cultured with P. chrysosporium or T. reesei presented higher activities of glucosidase (2-fold) and xylosidase (3-fold). By co-culturing low glucosidase-producing A. tubingensis with high glucosidase-producing T. reesei, Intasit et al. (Citation2023) demonstrated the enhanced biovalorization of palm empty fruit bunches. Several studies have shown that T. reesei co-cultures with both A. niger and other strains showed enhanced activities of some certain enzymes, while they might also cause reduced activities of other enzymes (Sperandio and Filho Citation2021). Shi et al. (Citation2021) selected five white-rot fungi strains among fifteen strains and compared their ligninolytic enzyme activities in single and co-cultivation. The results demonstrated that some co-cultures had synergistic effects on ligninolytic enzymes, some showed inhibitory effects, the others had negligible effects.
4.3. Process optimization
Whether it is the improvement of bioreactors or the optimisation of process parameters, it is very beneficial to optimise the existing fermentation technology before industrial production. Factors that have significant impacts on a general fermentation process include fermentation mode, strain, substrate, and process parameters (e.g. temperature, pH, agitation, dissolved oxygen, moisture). Usually, the enzyme-producing strain is selected based on the target lignocellulolytic enzyme and the composition of the agricultural waste to be utilised. Then the fermentation mode is decided according to the growth pattern of the strain. The most common goals are to minimise costs and maximise output by improving operational efficiency and shortening production periods. Parametric optimisation requires a proper design of experiment (Plackett–Burman design, Taguchi design, central composite design, and Box-Behnken design etc.) to establish validity, reliability, and replicability (Wang et al. Citation2020). Jana et al. (Citation2018) produced a multi-tolerant mannanase from copra meal by Aspergillus oryzae and optimised the moisture and pH of the SSF through central composite design, showing a 4.3-fold increase in mannanase production (434 U/gds). Due to the diversity and complexity of agricultural feedstocks, even using the same waste, it is possible to vary the culture composition by changing the biomass size, composition and supplements. Such changes can improve raw material utilisation and productivity without greatly changing the production process and cost. Thamvithayakorn et al. (Citation2019) performed Plackett-Burman design to screen medium components for cultivating Pseudolagarobasidium sp. on oil palm decanter cake. Compared to non-optimised production, the laccase, and the manganese peroxidase activities were enhanced by 2.59-fold and 1.94-fold, respectively. Gao et al. (Citation2023) planned a Box-Behnken design with substrate size, substrate ratio, and temperature, for the SSF of Chaetomium globosum on agricultural wastes. The optimised conditions greatly promoted the activities of several hydrolytic enzymes, namely xylanase (by 53.67%), endoglucanase (by 95.38%), and exoglucanase (by 33.43%). Further enhancement of production can be achieved by simultaneously optimising process conditions and medium components. One-factor-at-the-time analysis was used to optimise temperature, initial moisture, and supplemental nutrients for the fermentation of pineapple crowns by T. versicolor, resulting in a 6.7-fold increase in laccase activity (Backes et al. Citation2022). Taguchi design was applied to optimise raw material size, supplemented galactose and cupric sulphate concentration, inoculum size, temperature, and pH for laccase production on pineapple leaves by Marasmiellus palmivorus. Under optimised conditions, the yield of laccase increased 17.6-fold and reached the activity of 667.4 IU/mL (Chenthamarakshan et al. Citation2017).
5. Strain modifications of fungi
The fungi-derived lignocellulolytic enzymes from agricultural wastes contain numerous kinds of degrading enzymes, corresponding to the complex structural and chemical composition of the feedstocks. These enzyme systems work synergistically in the depolymerisation of lignocellulose. However, it is relatively rare for a wild-type strain to produce exactly the desired enzyme set with the activity levels required in industry. The high enzyme cost due to low efficiency and productivity remains a major barrier in its industrial application. Strain modification approaches can be targeted to improve the enzyme production level and enzyme selectivity in fungi.
5.1. Homologous expression
In homologous expression, proteins are derived from their original host, have better compatibility with the host, and are more likely to obtain higher expression levels. There is significant preference in the codon coding of genes in different species, and even in genes in the same species but with different functions. Thus, homologous expression requires fewer genetic modifications. The recombinant strains are not strictly considered transgenic, making them more acceptable for use, especially in food industry. Gao et al. (Citation2017) obtained an 9.1-fold and 51.5-fold increase in cellulase and xylanase from engineered Penicillium oxalicum (P. oxalicum) by overexpressing a mutated transcription factor XlnRA871V, a cellulase transcriptional activator ClrB and two major cellulase genes cbh1–2 and eg1. Separate engineering of transcriptional activators of P. oxalicum was reported to improve different sets of lignocellulolytic enzyme productions, and combined engineering of all three activators, ClrB, XlnR, and AraR, generated 3.1-fold to 51.0-fold increases in enzyme volumetric activities of exoglucanase, arabinofuranosidase, galactosidase, xylanase, and xylosidase (Gao et al. Citation2021). Zhou et al. (Citation2018) engineered Kluyveromyces marxianus by adding a T(−361)A mutation inside the inulinase promoter, deleting the AT-rich region inside 5’UTR (UTR∆A), and substituting P10L in the signal sequence. The recombinant strain showed up to 3-fold increased expressions of endoglucanase, xylanase, and mannanase. Liu et al. (Citation2017) deleted up to four genes, including cre-1, res-1, gh1–1, and alp-1, from the cellulase production pathway in M. thermophila with CRISPR/Cas9 genome-editing system, resulting in over 5-fold higher cellulolytic enzyme activities. Wang et al. (Citation2018) identified and modified a putative transcription regulator of cellulolytic enzymes, MHR1, in M. thermophila, leading to higher activities in exoglucanase (1.33-fold), endoglucanase (1.65-fold) and xylanase (1.48-fold).
5.2. Heterologous expression
Heterologous expression is the introduction of a gene from one species into another species. In most cases, the host strain selected for heterologous expression is generally regarded as safe and well-known for its fermentation and purification processes. These characteristics help to avoid potential risks caused by the use of pathogenic hosts, improve production efficiency, and save costs. On the other hand, the heterologously expressed proteins may lose activity or stability due to the significant differences in the codon coding between different species. Common approaches to address this issue are to optimise the codon composition without altering the amino acid sequence or expressing the gene in a heterologous host with a similar codon bias as the original host. It has been proposed that the lignocellulolytic enzyme genes from fungi can be cloned and expressed in both Escherichia coli and eukaryotic hosts such as Saccharomyces cerevisiae, Scheffersomyces stipitis (formerly Pichia stipitis) and filamentous fungi. listed some applications of heterologous expression for lignocellulolytic enzymes and properties of the recombinant enzymes.
Table 2. Heterologous expressions for lignocellulolytic enzymes and properties of the recombinant enzymes.
6. Conclusions and perspectives
Agricultural waste is a sustainable and promising feedstock for microbial enzyme production. Recent researchers have achieved great progress in producing fungi-derived lignocellulolytic enzymes from various agricultural wastes as well as optimising the composition and activities of the enzyme cocktails. Since the composition of different agricultural wastes varies greatly, identifying the suitable strain for a specific feedstock is the key to obtaining efficient enzyme production. In addition, the inhomogeneity and randomness of the agricultural residues may pose more challenges to large-scale production. Although biomass pretreatment is beneficial to high enzyme yield, it is critical to explore the balance between energy-intensive pretreatment and productivity improvement. Advanced fermentation technologies and modern strain modification techniques have brought fungal lignocellulolytic enzyme production to a new stage. However, multiple aspects of fungal enzyme production still require further study, particularly fungal metabolic pathways, process control, and enzyme selectivity. Overall, identifying and applying the appropriate production strategies will significantly promote fungi-derived lignocellulolytic enzyme production from agricultural wastes. The enhanced large-scale production of lignocellulolytic enzymes presents great potential for the industrial bioconversion of lignocellulosic materials into value-added compounds ().
Acknowledgments
The authors thank SUNY College of Environmental Science and Forestry for their support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Agnihotri S, Dutt D, Tyagi CH, Kumar A, Upadhyaya JS. 2010. Production and biochemical characterization of a novel cellulase-poor alkali-thermo-tolerant xylanase from Coprinellus disseminatus SW-1 NTCC 1165. World J Microbiol Biotechnol. 26(8):1349–1359. doi: 10.1007/s11274-010-0307-9.
- Ahmadi Khozani M, Emtiazi G, Aghaei SS, Ghasemi SM, Zolfaghari MR. 2021. Application of fungal laccase for heavy metals precipitation using tannin as a natural mediator. Int J Environ Sci Te. 18(8):2335–2344. doi: 10.1007/s13762-020-02992-7.
- Akpinar M, Ozturk Urek R. 2020. Decolorization and degradation potential of enhanced lignocellulolytic enzymes production by Pleurotus eryngii using cherry waste from industry. Biotechnol Appl Biochem. 67(5):760–773. doi: 10.1002/bab.1846.
- Akpinar M, Ozturk Urek R. 2022. Direct utilization of peach wastes for enhancements of lignocellulolytic enzymes productions by Pleurotus eryngii under solid-state fermentation conditions. Chem Pap. 76(11):6699–6712. doi: 10.1007/s11696-022-02356-0.
- An J, Xu W, Meng X, Chen G, Zhang W, Liu W. 2022. Biochemical characterization of a thermophilic exo-arabinanase from the filamentous fungus Rasamsonia emersonii. J Biosci Bioeng. 133(4):316–322. doi: 10.1016/j.jbiosc.2021.12.010.
- An Q, Liu ZY, Wang CR, Yang J, Chen SY, Chen X, YJ Z, Sen BL, Han ML. 2021. Laccase activity from Pleurotus ostreatus and Flammulina velutipes strains grown on agro-and forestry residues by solid-state fermentation. Bioresources. 16(4):7337–7354. doi: 10.15376/biores.16.4.7337-7354.
- Andlar M, Rezić T, Marđetko N, Kracher D, Ludwig R, Šantek B. 2018. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng Life Sci. 18(11):768–778. doi: 10.1002/elsc.201800039.
- Astolfi V, Astolfi AL, Mazutti MA, Rigo E, Di Luccio M, Camargo AF, Dalastra C, Kubeneck S, Fongaro G, Treichel H. 2019. Cellulolytic enzyme production from agricultural residues for biofuel purpose on circular economy approach. Bioprocess Biosyst Eng. 42(5):677–685. doi: 10.1007/s00449-019-02072-2.
- Awogbemi O, Von KD. 2022. Pretreatment techniques for agricultural waste. Case Studies Chem Environl Eng. 6:100229. doi:10.1016/j.cscee.2022.100229.
- Backes E, Kato CG, da Silva TBV, Uber TM, Pasquarelli DL, Bracht A, Peralta RM. 2022. Production of fungal laccase on pineapple waste and application in detoxification of malachite green. J Environ Sci Health B. 57(2):90–101. doi: 10.1080/03601234.2022.2025739.
- Barbosa FC, Silvello MA, Goldbeck R. 2020. Cellulase and oxidative enzymes: new approaches, challenges and perspectives on cellulose degradation for bioethanol production. Biotechnol Lett. 42(6):875–884. doi: 10.1007/s10529-020-02875-4.
- Ben Hmad I, Boudabbous M, Belghith H, Gargouri A. 2017. A novel ionic liquid-stable halophilic endoglucanase from Stachybotrys microspora. Process Biochem. 54:59–66. doi: 10.1016/j.procbio.2017.01.007.
- Benassi VM, de Lucas RC, Jorge JA, de LT de M PM. 2014. Screening of thermotolerant and thermophilic fungi aiming β-xylosidase and arabinanase production. Braz J Microbiol. 45(4):1459–1467. doi: 10.1590/S1517-83822014000400042.
- Chablé-Villacis R, Olguin-Maciel E, Toledano-Thompson T, Alzate-Gaviria L, Ruiz HA, Tapia-Tussell R. 2021. Enzymatic hydrolysis assisted with ligninocellulolytic enzymes from Trametes hirsuta produced by pineapple leaf waste bioconversion in solid-state fermentation. Biomass Conv Bioref. 13(10):9095–9106. doi: 10.1007/s13399-021-01851-w.
- Chapla D, Dholakiya S, Madamwar D, Shah A. 2013. Characterization of purified fungal endoxylanase and its application for production of value added food ingredient from agroresidues. Food Bioprod Process. 91(4):682–692. doi: 10.1016/j.fbp.2013.08.005.
- Chenthamarakshan A, Parambayil N, Miziriya N, Soumya PS, Lakshmi MSK, Ramgopal A, Dileep A, Nambisan P. 2017. Optimization of laccase production from Marasmiellus palmivorus LA1 by Taguchi method of design of experiments. BMC Biotechnol. 17(1):12. doi: 10.1186/s12896-017-0333-x.
- Chio C, Sain M, Qin W. 2019. Lignin utilization: A review of lignin depolymerization from various aspects. Renew Sust Energ Rev. 107:232–249. doi: 10.1016/j.rser.2019.03.008.
- Chukwuma OB, Rafatullah M, Tajarudin HA, Ismail N. 2020. Lignocellulolytic enzymes in biotechnological and industrial processes: A review. Sustainability (Switzerland). 12(18):7282. doi: 10.3390/su12187282.
- Claes A, Deparis Q, Foulquié-Moreno MR, Thevelein JM. 2020. Simultaneous secretion of seven lignocellulolytic enzymes by an industrial second-generation yeast strain enables efficient ethanol production from multiple polymeric substrates. Metab Eng. 59:131–141. doi: 10.1016/j.ymben.2020.02.004.
- da Rosa-Garzon NG, Laure HJ, Rosa JC, Cabral H. 2022. Valorization of agricultural residues using Myceliophthora thermophila as a platform for production of lignocellulolytic enzymes for cellulose saccharification. Biomass Bioenergy. 161:106452. doi: 10.1016/j.biombioe.2022.106452.
- Dave BR, Sudhir AP, Subramanian RB. 2015. Purification and properties of an endoglucanase from Thermoascus aurantiacus. Biotechnol Reports. 6:85–90. doi: 10.1016/j.btre.2014.11.004.
- de Almeida Antunes Ferraz JL, Souza LO, Soares GA, Coutinho JP, de Oliveira JR, Aguiar-Oliveira E, Franco M. 2018. Enzymatic saccharification of lignocellulosic residues using cellulolytic enzyme extract produced by Penicillium roqueforti ATCC 10110 cultivated on residue of yellow mombin fruit. Bioresour Technol. 248(PA):214–220. doi: 10.1016/j.biortech.2017.06.048.
- de Oliveira Júnior SD, dos Santos Gouvêa PR, de Aguiar LVB, Pessoa VA, dos Santos Cruz Costa CL, Chevreuil LR, Dedo BritoNascimento LB, dos Santos ES, Sales-Campos C. 2022. Production of lignocellulolytic enzymes and phenolic compounds by Lentinus strigosus from the amazon using solid-state fermentation (ssf) of guarana (Paullinia cupana) residue. Appl Biochem Biotechnol. 194(7):2882–2900. doi: 10.1007/s12010-022-03851-6.
- de Oliveira Simões LC, da Silva RR, de Oliveira Nascimento CE, Boscolo M, Gomes E, da Silva R. 2019. Purification and physicochemical characterization of a novel thermostable xylanase secreted by the fungus Myceliophthora heterothallica F.2.1.4. Appl Biochem Biotechnol. 188(4):991–1008. doi: 10.1007/s12010-019-02973-8.
- Dewan S. 2021. Global markets for enzymes in industrial applications [Internet]. Wellesley, MA; [accessed 2023 Jul 31]. https://www.bccresearch.com/market-research/biotechnology/global-markets-for-enzymes-in-industrial-applications.html
- Elegbede JA, Lateef A. 2018. Valorization of corn-cob by fungal isolates for production of xylanase in submerged and solid state fermentation media and potential biotechnological applications. Waste Biomass Valorization. 9(8):1273–1287. doi: 10.1007/s12649-017-9932-y.
- Escuder-Rodríguez JJ, González-Suarez M, deCastro ME, Saavedra-Bouza A, Becerra M, González-Siso MI. 2022. Characterization of a novel thermophilic metagenomic GH5 endoglucanase heterologously expressed in Escherichia coli and Saccharomyces cerevisiae. Biotechnol Biofuels. 15(1):76. doi: 10.1186/s13068-022-02172-4.
- Ezeilo UR, Wahab RA, Tin LC, Zakaria II, Huyop F, Mahat NA. 2020. Fungal-assisted valorization of raw oil palm leaves for production of cellulase and xylanase in solid state fermentation media. Waste Biomass Valorization. 11(7):3133–3149. doi: 10.1007/s12649-019-00653-6.
- Fatma S, Saleem A, Tabassum R. 2021. Wheat straw hydrolysis by using co-cultures of Trichoderma reesei and Monascus purpureus toward enhanced biodegradation of the lignocellulosic biomass in bioethanol biorefinery. Biomass Conv Bioref. 11(3):743–754. doi: 10.1007/s13399-020-00652-x.
- Favaro CP, Baraldi IJ, Casciatori FP, Farinas CS. 2020. β-mannanase production using coffee industry waste for application in soluble coffee processing. Biomolecules. 10(2):227. doi: 10.3390/biom10020227.
- Filipe D, Fernandes H, Castro C, Peres H, Oliva-Teles A, Belo I, Salgado JM. 2020. Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod Bioref. 14(1):78–91. doi: 10.1002/bbb.2073.
- Gao B, Ma Y, Xiao Y, Wang Y, Pan Y, Zhu D. 2023. Lignocellulolytic enzyme cocktail produced by plant endophytic Chaetomium globosum exhibits a capacity for high-efficient saccharification of raw rice straw. Ind Crops Prod. 196:116508. doi:10.1016/j.indcrop.2023.116508.
- Gao B, Xiao Y, Zhang Q, Sun J, Zhang Z, Zhu D. 2021. Concurrent production of glycyrrhetic acid 3-O-mono-β-d-glucuronide and lignocellulolytic enzymes by solid-state fermentation of a plant endophytic Chaetomium globosum. Bioresour Bioprocess. 8(1):88. doi: 10.1186/s40643-021-00441-y.
- Gao L, Gao F, Jiang X, Zhang C, Zhang D, Wang L, Wu G, Chen S. 2014. Biochemical characterization of a new β-glucosidase (Cel3E) from Penicillium piceum and its application in boosting lignocelluloses bioconversion and forming disaccharide inducers: New insights into the role of β-glucosidase. Process Biochem. 49(5):768–774. doi: 10.1016/j.procbio.2014.02.012.
- Gao L, He X, Guo Y, Wu Z, Zhao J, Liu G, Qu Y. 2021. Combinatorial engineering of transcriptional activators in Penicillium oxalicum for improved production of corn-fiber-degrading enzymes. J Agric Food Chem. 69(8):2539–2548. doi: 10.1021/acs.jafc.0c07659.
- Gao L, Li Z, Xia C, Qu Y, Liu M, Yang P, Yu L, Song X. 2017. Combining manipulation of transcription factors and overexpression of the target genes to enhance lignocellulolytic enzyme production in Penicillium oxalicum. Biotechnol Biofuels. 10(1):100. doi: 10.1186/s13068-017-0783-3.
- Garcia NFL, da Silva Santos FR, Gonçalves FA, da Paz MF, Fonseca GG, Leite RSR. 2015. Production of β-glucosidase on solid-state fermentation by Lichtheimia ramosa in agroindustrial residues: Characterization and catalytic properties of the enzymatic extract. Electron J Biotechn. 18(4):314–319. doi: 10.1016/j.ejbt.2015.05.007.
- Ge S, Chen X, Li D, Liu Z, Ouyang H, Peng W, Zhang Z. 2018. Hemicellulose structural changes during steam pretreatment and biogradation of Lentinus edodes. Arab J Chem. 11(6):771–781. doi: 10.1016/j.arabjc.2017.12.022.
- Gooruee R, Hojjati M, Behbahani BA, Shahbazi S, Askari H. 2022. Extracellular enzyme production by different species of Trichoderma fungus for lemon peel waste bioconversion. Biomass Conv Bioref. 11(1):162. doi: 10.1007/s13399-022-02626-7.
- Hosseini Koupaie E, Dahadha S, Bazyar Lakeh AA, Azizi A, Elbeshbishy E. 2019. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J Environ Manage. 233:774–784. doi:10.1016/j.jenvman.2018.09.106.
- Houfani AA, Anders N, Spiess AC, Baldrian P, Benallaoua S. 2020. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars– a review. Biomass Bioenergy. 134:105481. doi:10.1016/j.biombioe.2020.105481.
- Intasit R, Cheirsilp B, Louhasakul Y, Thongchul N. 2023. Enhanced biovalorization of palm biomass wastes as biodiesel feedstocks through integrated solid-state and submerged fermentations by fungal co-cultures. Bioresour Technol. 380:129105. doi:10.1016/J.BIORTECH.2023.129105.
- Intasit R, Cheirsilp B, Suyotha W, Boonsawang P. 2021. Synergistic production of highly active enzymatic cocktails from lignocellulosic palm wastes by sequential solid state-submerged fermentation and co-cultivation of different filamentous fungi. Biochem Eng J. 173:108086. doi:10.1016/j.bej.2021.108086.
- Jana UK, Suryawanshi RK, Prajapati BP, Soni H, Kango N. 2018. Production optimization and characterization of mannooligosaccharide generating Β-mannanase from Aspergillus oryzae. Bioresour Technol. 268:308–314. doi:10.1016/j.biortech.2018.07.143.
- Katsimpouras C, Dedes G, Thomaidis NS, Topakas E. 2019. A novel fungal GH30 xylanase with xylobiohydrolase auxiliary activity. Biotechnol Biofuels. 12(1):3619–3658. doi: 10.1186/s13068-019-1455-2.
- Khatoon N, Jamal A, Ali MI. 2019. Lignin peroxidase isoenzyme: a novel approach to biodegrade the toxic synthetic polymer waste. Environ Tech. 40(11):1366–1375. doi: 10.1080/09593330.2017.1422550.
- Kuo HW, Zeng JK, Wang PH, Chen WC. 2015. A novel exo-glucanase explored from a Meyerozyma sp. fungal strain. Adv Enzyme Res. 3(03):53–65. doi: 10.4236/aer.2015.33006.
- Leite P, Sousa D, Fernandes H, Ferreira M, Costa AR, Filipe D, Gonçalves M, Peres H, Belo I, Salgado JM. 2021. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr Opin Green Sustain Chem. 27:100407. doi:10.1016/j.cogsc.2020.100407.
- Li W, Yu J, Li Z, Yin WB. 2019. Rational design for fungal laccase production in the model host Aspergillus nidulans. Sci China Life Sci. 62(1):84–94. doi: 10.1007/s11427-017-9304-8.
- Liu E, Segato F, Wilkins MR. 2021. Fed-batch production of Thermothelomyces thermophilus lignin peroxidase using a recombinant Aspergillus nidulans strain in stirred-tank bioreactor. Bioresour Technol. 325:124700. doi:10.1016/j.biortech.2021.124700.
- Liu J, Yang J, Wang R, Liu L, Zhang Y, Bao H, Jang JM, Wang E, Yuan H. 2020. Comparative characterization of extracellular enzymes secreted by Phanerochaete chrysosporium during solid-state and submerged fermentation. Int J Biol Macromol. 152:288–294. doi:10.1016/j.ijbiomac.2020.02.256.
- Liu Q, Gao R, Li J, Lin L, Zhao J, Sun W, Tian C. 2017. Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol Biofuels. 10(1):1. doi: 10.1186/s13068-016-0693-9.
- Lu X, Li F, Zhou X, Hu J, Liu P. 2022. Biomass, lignocellulolytic enzyme production and lignocellulose degradation patterns by Auricularia auricula during solid state fermentation of corn stalk residues under different pretreatments. Food Chem. 384:132622. doi:10.1016/j.foodchem.2022.132622.
- Mahmood RT, Asad MJ, Mehboob N, Mushtaq M, Gulfraz M, Asgher M, Minhas NM, Hadri SH. 2013. Production, purification, and characterization of exoglucanase by Aspergillus fumigatus. Appl Biochem Biotechnol. 170(4):895–908. doi: 10.1007/s12010-013-0227-x.
- Majeke BM, García-Aparicio M, Biko OD, Viljoen-Bloom M, van Zyl WH, Görgens JF. 2020. Synergistic codon optimization and bioreactor cultivation toward enhanced secretion of fungal lignin peroxidase in Pichia pastoris: Enzymatic valorization of technical (industrial) lignins. Enzyme Microb Technol. 139:109593. doi: 10.1016/j.enzmictec.2020.109593.
- Malik K, Sharma P, Yang Y, Zhang P, Zhang L, Xing X, Yue J, Song Z, Nan L, Yujun S, et al. 2022. Lignocellulosic biomass for bioethanol: Insight into the advanced pretreatment and fermentation approaches. Ind Crops Prod. 188:115569. doi: 10.1016/j.indcrop.2022.115569.
- Martarello RD, Cunha L, Cardoso SL, de Freitas MM, Silveira D, Fonseca-Bazzo YM, Homem-de-Mello M, Filho EXF, Magalhães PO. 2019. Optimization and partial purification of beta-galactosidase production by Aspergillus niger isolated from Brazilian soils using soybean residue. AMB Express. 9(1):81. doi: 10.1186/s13568-019-0805-6.
- McNamara JT, Morgan JLW, Zimmer J. 2015. A molecular description of cellulose biosynthesis. Annu Rev Biochem. 84(1):895–921. doi: 10.1146/annurev-biochem-060614-033930.
- Méndez-Líter JA, Nieto-Domínguez M, Fernández De Toro B, González Santana A, Prieto A, Asensio JL, Cañada FJ, De Eugenio LI, Martínez MJ. 2020. A glucotolerant β-glucosidase from the fungus Talaromyces amestolkiae and its conversion into a glycosynthase for glycosylation of phenolic compounds. Microb Cell Fact. 19(1):127. doi: 10.1186/s12934-020-01386-1.
- Ming C, Dilokpimol A, Zou C, Liao W, Zhao L, Wang M, de Vries RP, Kang Y. 2019. The quest for fungal strains and their co-culture potential to improve enzymatic degradation of Chinese distillers’ grain and other agricultural wastes. Int Biodeterior Biodegradation. 144:104765. doi: 10.1016/j.ibiod.2019.104765.
- Moran-Aguilar MG, Costa-Trigo I, Calderón-Santoyo M, Domínguez JM, Aguilar-Uscanga MG. 2021. Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochem Eng J. 172:108060. doi:10.1016/j.bej.2021.108060.
- Mtibaà R, Barriuso J, de Eugenio L, Aranda E, Belbahri L, Nasri M, Martínez MJ, Mechichi T. 2018. Purification and characterization of a fungal laccase from the ascomycete Thielavia sp. and its role in the decolorization of a recalcitrant dye. Int J Biol Macromol. 120(PB):1744–1751. doi: 10.1016/j.ijbiomac.2018.09.175.
- Namnuch N, Thammasittirong A, Thammasittirong SNR. 2021. Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology. 12(2):119–127. doi: 10.1080/21501203.2020.1806938.
- Okado N, Sugi M, Kasamoto S, Mizuhashi F, Roberts A, Danielewska-Nikiel B, Sulaiman C, Pham S. 2020. Safety evaluation of arabinase (arabinan endo-1,5-α-L-arabinanase) from Aspergillus tubingensis. Food Sci Nutr. 8(1):456–478. doi: 10.1002/fsn3.1329.
- Orozco Colonia BS, Lorenci Woiciechowski A, Malanski R, Junior Letti LA, Soccol CR. 2019. Pulp improvement of oil palm empty fruit bunches associated to solid-state biopulping and biobleaching with xylanase and lignin peroxidase cocktail produced by Aspergillus sp. LPB-5. Bioresour Technol. 285:121361. doi:10.1016/j.biortech.2019.121361.
- Ozcirak Ergun S, Ozturk Urek R. 2017. Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann Agrar Sci. 15(2):273–277. doi: 10.1016/j.aasci.2017.04.003.
- Papadaki A, Kachrimanidou V, Papanikolaou S, Philippoussis A, Diamantopoulou P. 2019. Upgrading grape pomace through Pleurotus spp. Cultivation for the production of enzymes and fruiting bodies. Microorganisms. 7(7):207. doi: 10.3390/microorganisms7070207.
- Patel A, Divecha J, Shah A. 2021. Fomitopsis meliae CFA 2, a novel brown rot for endoglucanase: emphasis towards enhanced endoglucanase production by statistical approach. Mycology. 12(4):325–340. doi: 10.1080/21501203.2021.1918277.
- Perdani MS, Margaretha G, Sahlan M, Hermansyah H. 2020. Solid state fermentation method for production of laccase enzyme with bagasse, cornstalk and rice husk as substrates for adrenaline biosensor. Ener Rep. 6(S1):336–340. doi: 10.1016/j.egyr.2019.08.065.
- Qi J, Jia L, Liang Y, Luo B, Zhao R, Zhang C, Wen J, Zhou Y, Fan M, Xia Y. 2022. Fungi’s selectivity in the biodegradation of Dendrocalamus sinicus decayed by white and brown rot fungi. Ind Crops Prod. 188(PB):115726. doi: 10.1016/j.indcrop.2022.115726.
- Rajasree KP, Mathew GM, Pandey A, Sukumaran RK. 2013. Highly glucose tolerant β-glucosidase from Aspergillus unguis: NII 08123 for enhanced hydrolysis of biomass. J Ind Microbiol Biotechnol. 40(9):967–975. doi: 10.1007/s10295-013-1291-5.
- Ralph J, Lapierre C, Boerjan W. 2019. Lignin structure and its engineering. Curr Opin Biotechnol. 56:240–249. doi:10.1016/j.copbio.2019.02.019.
- Rao J, Lv Z, Chen G, Peng F. 2023. Hemicellulose: Structure, chemical modification, and application. Prog Polym Sci. 140:101675. doi:10.1016/j.progpolymsci.2023.101675.
- Raud M, Kikas T, Sippula O, Shurpali N. 2019. Potentials and challenges in lignocellulosic biofuel production technology. Renew Sust Energ Rev. 111:44–56. doi: 10.1016/j.rser.2019.05.020.
- Ravindran R, Williams GA, Jaiswal AK. 2019. Spent coffee waste as a potential media component for xylanase production and potential application in juice enrichment. Foods. 8(11):585. doi: 10.3390/foods8110585.
- Rekik H, Zaraî Jaouadi N, Bouacem K, Zenati B, Kourdali S, Badis A, Annane R, Bouanane-Darenfed A, Bejar S, Jaouadi B. 2019. Physical and enzymatic properties of a new manganese peroxidase from the white-rot fungus Trametes pubescens strain i8 for lignin biodegradation and textile-dyes biodecolorization. Int J Biol Macromol. 125:514–525. doi:10.1016/j.ijbiomac.2018.12.053.
- Rodríguez-Luna D, Ruiz HA, González-Morales S, Sandoval-Rangel A, Cabrera de la Fuente M, Charles-Rodríguez AV, Robledo-Olivo A. 2022. Recovery of melon residues (Cucumis melo) to produce lignocellulolytic enzymes. Biomass Convers Biorefin. 12(12):5915–5922. doi: 10.1007/s13399-020-01055-8.
- Rungrattanakasin B, Premjet S, Thanonkeo S, Klanrit P, Thanonkeo P. 2018. Cloning and expression of an endoglucanase gene from the thermotolerant fungus Aspergillus fumigatus DBiNU-1 in Kluyveromyces lactis. Braz J Microbiol. 49(3):647–655. doi: 10.1016/j.bjm.2017.10.001.
- Saini S, Sharma KK. 2021. Fungal lignocellulolytic enzymes and lignocellulose: A critical review on their contribution to multiproduct biorefinery and global biofuel research. Int J Biol Macromol. 193(PB):2304–2319. doi: 10.1016/j.ijbiomac.2021.11.063.
- Saldarriaga-Hernández S, Velasco-Ayala C, Leal-Isla Flores P, de Jesús Rostro-Alanis M, Parra-Saldivar R, Iqbal HMN, Carrillo-Nieves D. 2020. Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes. Int J Biol Macromol. 161:1099–1116. doi:10.1016/j.ijbiomac.2020.06.047.
- Saratale GD, Saratale RG, Ghodake GS, Jiang YY, Chang JS, Shin HS, Kumar G. 2017. Solid state fermentative lignocellulolytic enzymes production, characterization and its application in the saccharification of rice waste biomass for ethanol production: An integrated biotechnological approach. J Taiwan Inst Chem Eng. 76:51–58. doi:10.1016/J.JTICE.2017.03.027.
- Senthivelan T, Kanagaraj J, Panda RC, Narayani T. 2019. Screening and production of a potential extracellular fungal laccase from Penicillium chrysogenum: Media optimization by response surface methodology (RSM) and central composite rotatable design (CCRD). Biotechnol Reports. 23:e00344. doi: 10.1016/j.btre.2019.e00344.
- Shalaby ASG, Esawy MA, Hussein MDM. 2017. Comparative study between free and immobilized Penicillium chrysogenum mannanase: A local fungal isolate. J Appl Pharm Sci. 7(6):97–104. doi: 10.7324/JAPS.2017.70613.
- Sharma D, Garlapati VK, Goel G. 2016. Bioprocessing of wheat bran for the production of lignocellulolytic enzyme cocktail by Cotylidia pannosa under submerged conditions. Bioengineered. 7(2):88–97. doi: 10.1080/21655979.2016.1160190.
- Shi K, Liu Y, Chen P, Li Y. 2021. Contribution of lignin peroxidase, manganese peroxidase, and laccase in lignite degradation by mixed white-rot fungi. Waste Biomass Valorization. 12(7):3753–3763. doi: 10.1007/s12649-020-01275-z.
- Silva AFV, Santos LA, Valença RB, Porto TS, Da Motta Sobrinho MA, Gomes GJC, Jucá JFT, Santos AFMS. 2019. Cellulase production to obtain biogas from passion fruit (Passiflora edulis) peel waste hydrolysate. J Environ Chem Eng. 7(6):103510. doi: 10.1016/j.jece.2019.103510.
- Singh P, Jain P, Verma R, Jagadish RS. 2016. Characterization of lignin peroxidase from Paecilomyces species for decolorisation of pulp and paper mill effluent. J Sci Ind Res. 75(8):500–505.
- Sista Kameshwar AK, Qin W. 2018. Comparative study of genome-wide plant biomass-degrading CAZymes in white rot, brown rot and soft rot fungi. Mycology. 9(2):93–105. doi: 10.1080/21501203.2017.1419296.
- Sperandio GB, Filho EXF. 2021. An overview of Trichoderma reesei co-cultures for the production of lignocellulolytic enzymes. Appl Microbiol Biotechnol. 105(8):3019–3025. doi: 10.1007/s00253-021-11261-7.
- Sugano J, Maina N, Wallenius J, Hildén K. 2021. Enhanced lignocellulolytic enzyme activities on hardwood and softwood during interspecific interactions of white-and brown-rot fungi. J Fungus. 7(4):265. doi: 10.3390/jof7040265.
- Sunardi TJ, Ishiguri F, Ohshima J, Iizuka K, Yokota S. 2016. Changes in lignocellulolytic enzyme activity during the degradation of Picea jezoensis wood by the white-rot fungus Porodaedalea pini. Int Biodeterior Biodegradation. 110:108–112. doi:10.1016/j.ibiod.2016.02.022.
- Sung HJ, Khan MF, Kim YH. 2019. Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int J Biol Macromol. 136:20–26. doi:10.1016/j.ijbiomac.2019.06.026.
- Thamvithayakorn P, Phosri C, Pisutpaisal N, Krajangsang S, Whalley AJS, Suwannasai N. 2019. Utilization of oil palm decanter cake for valuable laccase and manganese peroxidase enzyme production from a novel white-rot fungus. Pseudolagarobasidium. 9(11):417. sp. PP17-33. 3 Biotech. doi:10.1007/s13205-019-1945-8.
- Ul HI, Akram F. 2019. Enhanced production, overexpression and characterization of a hyperthermophilic multimodular GH family 2 β glucuronidase (TpGUS) cloned from Thermotoga petrophila RKU-1T in a mesophilic host. Int J Biol Macromol. 123:1132–1142. doi:10.1016/j.ijbiomac.2018.11.189.
- Vázquez-Montoya EL, Castro-Ochoa LD, Maldonado-Mendoza IE, Luna-Suárez S, Castro-Martínez C. 2020. Moringa straw as cellulase production inducer and cellulolytic fungi source. Rev Argent Microbiol. 52(1):4–12. doi: 10.1016/J.RAM.2019.02.005.
- Velasco J, Oliva B, Gonçalves AL, Lima AS, Ferreira G, França BA, Mulinari EJ, Gonçalves TA, Squina FM, Kadowaki MAS, et al. 2020. Functional characterization of a novel thermophilic exo-arabinanase from Thermothielavioides terrestris. Appl Microbiol Biotechnol. 104(19):8309–8326. doi: 10.1007/s00253-020-10806-6.
- Vidya B, Palaniswamy M, Angayarkanni J, Ayub Nawaz K, Thandeeswaran M, Krishna Chaithanya K, Tekluu B, Muthusamy K, Gopalakrishnan VK. 2020. Purification and characterization of β-galactosidase from newly isolated Aspergillus terreus (KUBCF1306) and evaluating its efficacy on breast cancer cell line (MCF-7). Bioorg Chem. 94:103442. doi:10.1016/j.bioorg.2019.103442.
- Wang J, Gong Y, Zhao S, Liu G. 2018. A new regulator of cellulase and xylanase in the thermophilic fungus Myceliophthora thermophila strain ATCC 42464. 3 Biotech. 8(3):160. doi: 10.1007/s13205-017-1069-y.
- Wang J, Huang J, Laffend H, Jiang S, Zhang J, Ning Y, Fang M, Liu S. 2020. Optimization of immobilized Lactobacillus pentosus cell fermentation for lactic acid production. Bioresour Bioprocess. 7(1):15. doi: 10.1186/s40643-020-00305-x.
- Wang J, Liu S, Huang J, Cui R, Xu Y, Song Z. 2023. Genetic engineering strategies for sustainable polyhydroxyalkanoate (PHA) production from carbon-rich wastes. Environ Technol Inno. 30:103069. doi: 10.1016/j.eti.2023.103069.
- Wang J, Liu S, Huang J, Qu Z. 2021. A review on polyhydroxyalkanoate production from agricultural waste Biomass: Development, Advances, circular Approach, and challenges. Bioresource Techno. 342:126008. doi: 10.1016/j.biortech.2021.126008.
- Xia C, Li Z, Xu Y, Yang P, Gao L, Yan Q, Li S, Wang Y, Qu Y, Song X. 2019. Introduction of heterologous transcription factors and their target genes into Penicillium oxalicum leads to increased lignocellulolytic enzyme production. Appl Microbiol Biotechnol. 103(6):2675–2687. doi: 10.1007/s00253-018-09612-y.
- Xia Y, Yang L, Xia L. 2018. High-level production of a fungal β-glucosidase with application potentials in the cost-effective production of Trichoderma reesei cellulase. Process Biochem. 70:55–60. doi: 10.1016/j.procbio.2018.03.031.
- Xu G, Wang J, Yin Q, Fang W, Xiao Y, Fang Z. 2019. Expression of a thermo- and alkali-philic fungal laccase in Pichia pastoris and its application. Protein Expr Purif. 154:16–24. doi:10.1016/j.pep.2018.09.015.
- Zhang H, Zhang J, Zhang X, Geng A. 2018. Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching. Process Biochem. 66:222–229. doi: 10.1016/j.procbio.2017.12.011.
- Zhou J, Zhu P, Hu X, Lu H, Yu Y. 2018. Improved secretory expression of lignocellulolytic enzymes in Kluyveromyces marxianus by promoter and signal sequence engineering. Biotechnol Biofuels. 11(1):235. doi: 10.1186/s13068-018-1232-7.
- Zou S, Liu G, Kaleem I, Li C. 2013. Purification and characterization of a highly selective glycyrrhizin-hydrolyzing β-glucuronidase from Penicillium purpurogenum Li-3. Process Biochem. 48(2):358–363. doi: 10.1016/j.procbio.2012.12.008.