 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
The aim of this study was to describe and analyse the variety of respiratory appearances in Long COVID subjects who were not hospitalised during the acute phase of the infection.
Methods
A consecutive series of 60 subjects participated 10.8 months (SD 4.5) after the acute phase of the infection. Respiratory function was tested concerning lung volumes, expiratory flow, muscle strength, physical capacity including concurrent oxygen saturation, chest expansion, lung sounds, pain and breathing pattern. Differences between those with or without positive test and duration of symptoms more or less than 6 months were analysed with T-test, Chi-square test and Fisher’s exact test.
Results
Decreased forced vital capacity was found in 6/60 (10%), and forced expiratory volume in 1 s and 7/60 (12%), low maximal inspiratory pressure in 38/58 (54%) and low maximal expiratory pressure in 10/58 (17%). Decreased physical capacity was registered in 36/52 (69%), and thoracic expansion in 26/46 (56%). Pathologic lung sounds had 15/58 (26%) and six patients desaturated during the test of physical capacity. A majority (36/58, 67%) presented pain in the ribcage. All but three patients (95%) showed a dysfunctional breathing pattern in sitting and standing. Only poor and fair correlations were found between age, duration and level of physical capacity compared to spirometry, respiratory muscle strength and thoracic expansion.
Conclusion
Abnormal breathing pattern and respiratory movements as well as pain, and reduced lung volumes, flow, respiratory muscle strength, physical capacity and thoracic expansion may be involved in Long COVID. The breathing symptoms should therefore be looked for in a wider picture beyond spirometry and oximetry.
Introduction
Covid-19 is a massive threat to public health with more than 169 million registered cases worldwide and more than 1,170,000 cases in Sweden at the end of October 2021 [Citation1,Citation2]. It was early known that this new virus, called SARS-CoV-2, may cause different symptoms in a wide range from very light to major ones [Citation3]. The main cause of mortality is respiratory distress syndrome, sometimes simultaneously with multi-organ failure [Citation4].
Around 10% of those being hospitalised remain unwell beyond 3 weeks, a smaller proportion for months [Citation5,Citation6]. Not only physical, but also cognitive and psychosocial sequelae are common, which is a great challenge to allied health professionals and rehabilitation teams [Citation7]. Long COVID is described as ‘a varying combination of overlapping symptoms’ [Citation8]. Due to a lack of a clear definition and to different study methods, the incidence varies [Citation6,Citation9–12]. The symptoms have also been reported to vary in time within individuals [Citation5]. Most common ongoing symptoms are fatigue, palpitations, dyspnoea, chest pain, chest pressure, headache and joint pain [Citation8,Citation11,Citation13,Citation14]. Some subjects suffer from continuous or fluctuating fever over a long time [Citation6]. This may result into reverses or setbacks, and affects physical activity and rehabilitation.
Long COVID is not associated with the severity of the initial infection, and the course of the condition is often fluctuating; just as they feel they are getting better the symptoms return [Citation5,Citation14]. A thorough assessment and an individual, progressive treatment plan which focuses on function, disability, and return to participation in society may help each subject to maximise their function and quality of life [Citation14–16]. Long COVID is seen in previously hospitalised subjects but also in subjects who did not require hospitalisation [Citation17–19]. It can appear one to several months after recovering from the acute phase and may consist of persistent symptoms or new ones [Citation5,Citation14]. The pathophysiology of this condition is not yet known. Long COVID has been suggested to require a whole-subject perspective and multi-disciplinary, multispecialty approaches to assessment and management [Citation5,Citation6,Citation14,Citation15,Citation20].
The World Health Organisation (WHO) has, among other organisations, written general and specialised clinical practice guidelines [Citation21,Citation22]. Most of these publications are based on subjects during or after discharge from hospital care, less has been written about the rehabilitation needs or outcomes in the long term. To an increasing extent publications are based upon direct observation of the actual trajectories of subjects with the disease, the individual lasting effects after the acute phase of the disease are yet to be revealed [Citation14,Citation23]. As respiratory symptoms are common in Long COVID, studies are needed to explore their possible origin [Citation24]. It is necessary to determine the reason or source of chest pain, dyspnoea or hypoxia to prevent harm and to guide physical activity and exercise in an appropriate way [Citation25].
The aim of this study is therefore to describe respiratory appearances in Long COVID subjects who were not hospitalised during the acute phase of the infection.
Methods
This is a prospective cross-sectional study where a consecutive series of 60 unselected subjects (nine men) participated. They had all been referred by the local general practitioner or by the Long-COVID team to the physiotherapy outpatient clinic at the Sahlgrenska University Hospital, because of remaining symptoms after Covid-19 infection which had been confirmed by tests or clinically [Citation26]. The outpatient clinic is organised with physiotherapists who are certified specialists in respiration. The patients were on their first visit in the clinic from August 2020 until September 2021. None of the patients declined participation.
The Swedish Ethical Review Authority approved the study (registration number 2020-02149, 2020‐03215 and 2021‐01101). The subjects were included after oral and written information and written consent.
The mean age of the referred subjects was 44.5 years (SD 9.4), of whom 48 (80%) were 30–55 years old. The BMI of the whole cohort was 24.7 kg/m2 (SD 4.9), 13 of them (22%) were defined as overweight (BMI 25–30 kg/m2) and 6 (10%) as obese (BMI > 30 kg/m2).
During the first visit at the physiotherapy outpatient clinic, the subjects filled in following questionnaires:
Degree of functional limitation
The degree of functional limitation on the disease specific scale was rated by the Post-Covid Functional Status (PCFS) [Citation14]. The scale is ranged from 0 (no limitation) to 4 (severe limitation in personal life).
Respiratory symptoms
Their remaining respiratory symptoms were rated on the COPD Assessment Test (CAT) scale from 0 to 40, where a higher number indicates more symptoms [Citation27]. To improve the interpretation minor additions were made to three of the eight statements. In question number two ‘phlegm in the chest’ the word ‘lungs’ was added. In statement number five and eight ‘because of my lung condition’ was added in accordance with statement number six in the original version.
Physical activity level
Their physical activity level was estimated according to Physical Activity Level Scale by Frändin and Grimby [Citation28]. It is a scale where duration and intensity of physical activity and training is assessed from 1 (sedentary) to 6 (intensive physical training > 3 h/week).
In addition, several tests were performed to evaluate different aspects of respiratory symptoms. No standardised protocol was followed as only relevant tests for each subject were undertaken. Following tests were included.
Spirometry
In a seated position, feet on the floor, and with a nose clip, each subject performed a spirometry using a portable ultrasonic spirometer (Easy One ndd, Medical Technologies, Switzerland). Forced Vital Capacity (FVC), Forced Expiratory Volume in one second (FEV1) and Peak Expiratory Flow (PEF) were measured following the European Respiratory Societies recommendations [Citation29,Citation30]. The results were analysed as percentage of predicted in reference values according to gender, age and height according to the reference equations for spirometry [Citation31].
Respiratory muscle strength
Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP), expressed in cm H2O, were measured in a standardised manner using a micro-RPM (Care Fusion, Yorba Linda, CA, USA). In a seated position, feet on the floor, and with a nose clip, subjects were asked to perform a maximal inspiration from residual volume to measure MIP, MEP while performing a maximal expiration from total lung capacity [Citation32]. Three manoeuvres of each test were performed, interspaced by rest. Normal values and lower limit of normal for MIP were defined according to Sclauser Pessoa et al. [Citation33], and for MEP by Bissett et al. [Citation34].
Physical capacity measured by six minutes’ walk test or sit-to-stand test
The six minutes’ walk test (6MWT) was performed indoor in a corridor with a path of 30 m in a standardised manner [Citation35]. In this trial, distance walked, and oxygen saturation registered before and immediately after the test was reported. Predicted walking distance and lower limit of normal was defined according to Enright [Citation36]. Physical capacity was also measured by sit-to-stand test, where subjects were instructed to, for one minute, rise and sit from a 45 cm high chair in a standardised manner [Citation37]. The test was performed with feet on the floor and arms crossed over the chest. In this trial, number of rises was reported, and oxygen saturation was registered before and immediately after the test. Predicted number of rises was defined according to Strassman et al. [Citation37]. As no lower limit of normal was found, a value below 80% of predicted was defined as decreased. Oxygen saturation was measured by a pulse oximeter (Ras-57, Masimo Corporation, Irvine CA, USA).
Chest mobility by thoracic expansion
Thoracic expansion was assessed using a measuring tape (marked in mm) around the circumference at the level of the Xiphoid Process [Citation38]. The test was performed in standing position with the hands on the head. To measure the maximal movements, instructions were given as follows: ‘Breathe in maximally and make yourself as big as possible’ and ‘Breathe out maximally and make yourself as small as possible’ [Citation39]. As no lower limit of normal was found, a value below 80% of predicted was defined as decreased.
Respiratory movement
Respiratory movements were recorded standardised with the Respiratory Movement-Measuring Instrument (RMMI, ReMo, Reykjavik, Iceland) [Citation40]. The measurements registered as real-time bilateral changes in anterior posterior diameter, included upper and lower thoracic and abdominal movement during tidal volume and deep breathing [Citation40]. The subjects were not aware of when the data were recorded for tidal volume breathing. During the deep-breathing manoeuvre, the participants were instructed to take maximal breaths. Data were collected during 30 s for the tidal volume breathing and during 1 min for the deep breathing manoeuvres. Reference values were based on the trial by Ragnarsdottir et al. [Citation40].
Breathing pattern
Breathing pattern was assessed by using ocular evaluation of the upper and lower thorax, and abdominal movements in supine, sitting and standing position. Normal breathing pattern was defined as predominantly abdominal and lower thoracic movements during tidal volume breathing in sitting and standing.
Lung sounds by auscultation
Both lungs were assessed by standardised auscultation using a stethoscope (Littman, Select. Littman, 3 M Health care, Borken, Germany) during tidal volume breathing and forced expirations. Apical, mid, and basal parts of the lungs were assessed dorsally and apical parts ventrally. Sounds of secretions and obstruction were registered.
Pain in the chest by palpation
Musculoskeletal pain was standardised assessed bilaterally in all sternocostal joints and anterior intercostal muscles by manual palpation. Pain was registered as yes/no.
Statistics
Results were presented per subject and mean ± standard deviation or number of patients (%). Data were presented as percent of predicted values. In variables where lower level of normal was missing, results of <80% of predicted values were defined as reduced.
Analyses between those with duration of symptoms more or less than 6 months, those having performed a Covid-19 test or not, between men and women and between those with and without pain in the ribcage, were all performed by T-test, Chi-square test and Fisher’s exact test. A probability <0.05 was defined as significant.
Correlation was analysed on three variables: age, duration of symptoms and physical capacity, to spirometry, respiratory muscle strength and thoracic mobility. Correlation was defined as poor (r < 0.20), fair (r = 0.21–0.40), moderate (r = 0.41–0.60), good (r = 0.61–0.80) and very good (r = 0.81–1.00) [Citation41].
Results
The mean time since the acute phase of the infection was 10.8 months (SD 4.5, range 3–18). Of the cohort 32 (53%) had been tested positive for Covid-19. The remaining were, by the patients’ general practitioners or the hospital, clinically considered to having had a corona infection according to the Swedish Board of Health and Welfare [Citation26] but were never tested by the health care. None of them had been hospitalised during the acute phase of the disease, although several had visited the emergency department but been rejected.
After the acute phase, the subjects had had different remaining respiratory symptoms. At the time of the visit in the physiotherapy outpatient clinic, 54 (90%) had other symptoms; cognitive impairment (n = 37, 62%), fatigue (n = 46, 77%) and episodes of tachycardia (n = 35, 58%). Three patients (5%) were smokers and 7 (12%) had a previously diagnosed pulmonary disease (asthma n = 6, chronic bronchitis n = 1). Their level of physical activity according to Physical Activity Level Scale [Citation28] before the infection was ‘sedentary’ (n = 2, 3%), ‘some light’ (n = 37, 62%), ‘regular physical activity and training’ (n = 17, 28%) and ‘regular and physical training for competitive sports’ (n = 4, 7%).
Degree of functional limitation by PCFS was by 3 subjects (5%) rated ‘negligible’, 21 (35%) rated ‘slight’, 28 subjects (47%) rated ‘moderate’ and 8 (13%) rated ‘severe functional limitation’. Their remaining respiratory symptoms were in median 20 (range 3–31) on the modified CAT scale. Feeling of tightness around the chest was reported by 44 subjects (73%). The sensation was described as: something (weights of different kind) pressing on the chest, feeling of a tight armour/girdle/belt/bra around the chest or sensations of strangulation.
Results of the tests, in total and divided in groups based on duration of symptoms and if they had a positive test or not, are presented in and .
Figure 1. Level of symptoms in Long COVID by grouping the results (in percent of predicted values). The diagram contains six graphs showing the results in Forced Vital Capacity (FVC), Forced Expiratory Volume in One second (FEV1), Maximal Inspiratory Pressure (MIP), Maximal Expiratory Pressure (MEP), physical capacity (Phys cap), and thoracic expansion (Thx exp).
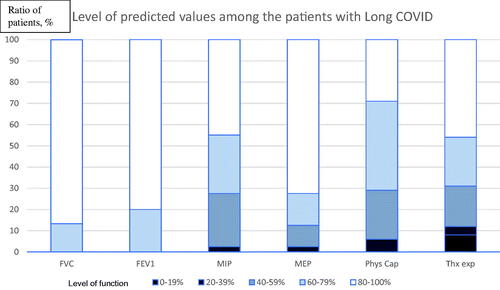
Table 1. Results of included tests. Results are presented as percent predicted (mean and standard deviation) or n (%).
Details, per patient, are presented in supplement. Concerning spirometry six of the subjects had a reduced FVC and seven reduced FEV1. PEF was below normal values in one subject (data not shown). Sixty-four percent had reduced MIP and 17% reduced MEP. Physical capacity (6MWT n = 14 and TST n = 38) was reduced in 69% of the subjects, desaturation during the test (SpO2 < 92) was found in 10%. Thoracic expansion was reduced in 26% of the subjects, and of those, four subjects showed <20% of the predicted value. Secretions were reported during auscultation in one and ronchi was detected in 15 of the subjects. Pain was found in 67% of the subjects in either the sternocostal joints, the intercostal muscles, or both. Breathing pattern was found divergent in 57 of the 60 subjects (95%) and most common was breathing predominantly in the upper and lower thoracic level with no or minor abdominal movement (diaphragmatic breathing) in sitting and standing (see ).
Table 2. Respiratory movements in patients undergoing test with RMMI. Mean of both sides.
Duration of symptoms of more or less than 6 months did not had any impact on evaluated symptoms or signs, but in FVC (difference of 2.6%, p = 0.042) (). Neither were there any differences between groups when divided into those who had a positive Covid-19 test or not. Differences between men and women (data not shown) were minor except that the women had significantly lower respiratory muscle strength (MIP women 73.3 ± 25.7, men 100.4 ± 27.3% predicted, p = 0.37), MEP women 92.1 ± 7.4, men 135.4 ± 9.3% predicted, p < 0.001). Women also more often had pain in the intercostal joints (p = 0.035) and muscles (p = 0.029). No significant difference was seen in breathing pattern between sexes. Subjects with musculoskeletal chest pain had significantly lower MEP (94.9 ± 32.7%) and physical capacity (67.9 ± 20.9%) compared to those with no pain (MEP 113.3 ± 24.3% and physical capacity 86.8 ± 28.1%, p = 0.042 and p = 0.011).
Respiratory movements, measured in supine, were reduced in one or several diode positions in 22 of the 38 tested subjects (58%) during tidal volume breathing and in ten (31%) during deep breathing. One of the subjects had the larger movement in upper thoracic region than in the abdomen during tidal volume breathing and 19 (50%) during deep breathing.
Age had a fair correlation to FEV1 (% predicted) (r= −0.269, p = 0.038) but poor to FVC (r= −0.168, p = 0.198), respiratory muscle strength (r > 0.106, p = 0.424) and thoracic mobility (r= −0.085, p = 0.574). Duration of symptoms had poor correlation to spirometry, muscle strength and thoracic mobility (r < 0.2, p= ns). Physical capacity had poor correlation to spirometry, and respiratory muscle strength (r < 0.2, p= ns) and fair to thoracic mobility (r = 0.298, p = 0.052).
Discussion
Normally, when a viral infection subsides, the infected usually return to full recovery even if it may take time. In patients with Long COVID, prolonged respiratory impairments have been reported after hospitalisation with and without intensive care [Citation15]. But there is less knowledge about remaining symptoms among those who were never hospitalised in the acute phase. Interestingly, some subjects in our study had a very mild initial phase, but they still present long-term/return of heterogeneous symptoms, as reported in earlier trials [Citation14,Citation17,Citation24].
The results of this trial indicate that most of the subjects have lung volumes (FVC and FEV1) or flow (PEF) within normal values when tested. As well as spirometry, different tests of physical capacity in combination with a measure of the oxygen saturation are often clinically used. Several of our subjects had reduced physical capacity but did not desaturate during the test. However, the subjects still reported breathing difficulties and were bothered by various other symptoms which are not always obvious to interpret and measure. Descriptions of symptoms as chest pain, ‘lung burn’, shortness of breath and inability to take a deep breath, have sometimes been met with disbelief or lack of understanding from the care [Citation5]. With an increased battery of tests and examinations we were able to detect possible reasons to the patients’ symptoms.
In contrary to normal spirometry results, respiratory muscle strength was reduced in most of our subjects. However, MIP and MEP are less often measured in clinic. Regardless, respiratory muscle strength is crucial in many categories of subjects, as in chronic obstructive pulmonary disease, neuromuscular diseases, and heart failure [Citation42]. It seems like MIP and MEP are very important to measure also in subjects with Long COVID with respiratory symptoms.
Thoracic expansion was measured to obtain information which could be useful to understand the experience of tightness of the chest and it was reduced in 56% of our study-population. This is easily measured and may give important information about chest mobility even if its reliability has been questioned [Citation43]. However, only three physiotherapists, all specialists in respiration, were involved in the measurements which increase the reliability. The difference of the circumference measures the maximal rib-cage movement [Citation38]. In this trial, we also measured respiratory movement with an objective specific equipment (RMMI). The movement in supine was abnormal during tidal volume breathing in one or several diode positions in 50% of the measured subjects during deep breathing. Even though almost the same proportion of subjects had limitations in chest mobility, these two measurements were not performed in the same position (standing vs lying supine) and results are therefore not transferable. Breathing pattern, assessed subjectively, was divergent in almost all subjects, despite of normal or reduced thoracic expansion and respiratory movement. The subjects’ experience of chest tightness and dyspnoea may have an origin in the dysfunctional breathing pattern. Possible reasons may be reduced respiratory muscle strength and a dysfunctional diaphragm. The reason behind this is not known but may be on cellular level or caused by dysfunctional autonomy, which also has been discussed in a couple of publications [Citation44,Citation45]. The differences in respiratory movements between tidal volume and deep breathing may be the involuntary, autonomously controlled tidal volume breathing when the subjects did not know they were tested, and the voluntary manoeuvres made during the deep breathing. The chest tightness that subjects experience may be connected to the pain in the sternocostal joints and intercostal muscles. The chest is a very tight construction with a limited range of motion, therefore susceptible for internal and external influences.
When further analysing the results, no clinically relevant significant differences were found between subjects with longer (>6 months) than shorter duration of symptoms. An interpretation of this is that symptoms may sustain if not treated. Neither were there any differences between those who had showed a positive Covid-19 test or not. During the first wave of the pandemic, patients with obvious symptoms were refused to be tested due to lack of test kits. According to the Swedish Board of Health and Welfare [Citation26] such patients should clinically be considered to having had a corona infection and this definition is used in this study.
Women showed more impact on respiratory muscle strength and had more pain. The reason for this is not known. In addition, the tested subjects with pain in the ribcage had significantly lower MEP and physical capacity. To define the individual problem and evaluation factors for the treatment it seems important to widen the test battery for patients with a variety of symptoms.
There are some strengths of this study. It was performed in a university hospital on a consecutive series of unselected patient who were referred to the clinic by their local general practitioner or the Long-COVID team. All subjects had remaining respiratory symptoms and were measured by one of three certified specialised physiotherapists and data was collected prospectively. Another strength is the access to various equipment to measure different aspects of respiration as respiratory movement and respiratory muscle strength. There are also limitations. Some of the measurements, as auscultation and evaluation of breathing pattern, were subjectively assessed. Another limitation is that the subjects were not tested concerning diffusion capacity, or circulatory complications in the lungs, neither were there any images of the lungs included in the protocol. However, only 10% desaturated during physical capacity test which indicate a normal diffusion capacity of the majority. To be able to analyse the study-sample as one group, normal- and reference values are given where differences such as sex, age and height are considered. To be able to evaluate normality of the results available ‘lower limit of normal’ was used. Some of the tests do not have such values and values <80% was defined as abnormal. However, the reductions did most often exceed 30% which indicates an even larger deterioration. In addition, it is a limitation that we have no pre-covid test-results to compare current results to, but this is a due to the nature of the disease. The material is also skew concerning sex. Of the patients who have been hospitalised because of a Covid-19 infection the majority is men, but among those who were not hospitalised it was the opposite. The ratio between men and women are reflecting the situation in Sweden [Citation46].
World Physiotherapy [Citation25] emphasises that it is of importance to search for reasons or sources of the remaining respiratory symptoms and to monitor identified hyperventilation and breathing pattern disorders. Our results could constitute a base for specialist respiratory physiotherapists to tailor subject-centred interventions to relieve or cure remaining symptoms. The results from this study give valuable information of such symptoms and may be a starting point of further investigations and for clinical decisions concerning interventions.
In conclusion, respiratory symptoms in subjects with Long COVID may have several causes. Among other, reduction in lung volume, flow, respiratory muscle strength, physical capacity, chest mobility, respiratory movements, breathing pattern and pain may be involved. To capture a more whole picture, breathing symptoms in Long COVID should therefore be searched beyond spirometry and oximetry.
Author contributions
M.F.O., L.L. E.L.J.: Data collection, M.F.O., L.L., M.N.B., E.L.J.: Conceptualisation, methodology and writing.
Supplemental Material
Download MS Word (36.7 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analysed during this study were included in this published article and its supplementary information file.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- WHO [Internet]. Weekly epidemiological update on COVID 19 – 31 May 2021. [cited May 20, 2021. Available from: https://wwwwhoint/pubications/m/item/weekly-epidemiological-update-on-COVID-19
- Public Health Authority in Sweden (Folkhälsomyndigheten) [Internet]. Daily update on Covid – 31 October 2021. Available from: https://fohm.maps.arcgis.com/apps/opsdashboard/index.html
- Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Res Sq. 2021.
- Rajan S, Khunti K, Alwan N, et al. In the wake of the pandemic: preparing for Long COVID. Copenhagen (Denmark): European Observatory Policy Briefs; 2021.
- Ladds E, Rushforth A, Wieringa S, et al. Persistent symptoms after Covid-19: qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv Res. 2020;20(1):1144.
- Greenhalgh T, Knight M, A'Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026.
- Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months After hospital discharge. JAMA Netw Open. 2021;4(1):e2036142.
- Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med. 2021;268:113426.
- Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems Network - United States, March-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998.
- Sudre CH, Keshet A, Graham MS, et al. Anosmia and other SARS-CoV-2 positive test-associated symptoms, across three national, digital surveillance platforms as the COVID-19 pandemic and response unfolded: an observation study. medRxiv. 2020.
- Carfi A, Bernabei R, Landi F, et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605.
- Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA. 2021;325(19):2015–2016.
- Sheehy LM. Considerations for postacute rehabilitation for survivors of COVID-19. JMIR Public Health Surveill. 2020;6(2):e19462.
- Jacobson KB, Rao M, Bonilla H, et al. Patients with uncomplicated COVID-19 have long-term persistent symptoms and functional impairment similar to patients with severe COVID-19: a cautionary tale during a global pandemic. Clin Infect Dis. 2021.
- O’Sullivan O, Barker-Davies RM, Thompson K, et al. Rehabilitation post-COVID-19: cross-sectional observations using the Stanford Hall remote assessment tool. BMJ Mil Health. 2021.
- Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á, et al. Post-COVID-19 syndrome and the potential benefits of exercise. IJERPH. 2021;18(10):5329.
- Vaes AW, Goertz YMJ, Van Herck M, et al. Recovery from COVID-19: a sprint or marathon? 6-month follow-up data from online long COVID-19 support group members. ERJ Open Res. 2021;7(2):00141–2021.
- Townsend L, Dowds J, O'Brien K, et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18(6):997–1003.
- Geddes L. The enduring grip of Covid-19. New Sci. 2020;246(3288):34–38.
- Gemelli NA, Boccalatte LA. Expected results from a predictable phenomenon: an approach to education in times of pandemics. Disaster Med Public Health Prep. 2020.
- Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66(2):73–82.
- Spruit MA, Holland AE, Singh SJ, et al. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society and American Thoracic Society-coordinated International Task Force. Eur Respir J. 2020;56(6):2002197.
- O'Sullivan O. Long-term sequelae following previous coronavirus epidemics. Clin Med (Lond). 2021;21(1):e68–e70.
- Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122.
- Safe rehabilitation approaches for people living with Long COVID: Physical Activity and Exercise [Internet]. Available from: https://world.physio/sites/default/files/2021-06/Briefing-Paper-9-Long-Covid-FINAL-2021.pdf
- Swedish Board of Health and Welfare (Socialstyrelsen). Postcovid – kvarstående eller sena symtom efter covid-19 Stöd till beslutsfattare och personal i hälso- och sjukvården (del 2). Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/ovrigt/2021-4-7351.pdf
- Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654.
- Grimby G, Borjesson M, Jonsdottir IH, et al. The “Saltin-Grimby Physical Activity Level Scale” and its application to health research. Scand J Med Sci Sports. 2015;25(Suppl 4):119–125.
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40.
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343.
- American Thoracic Society. European Respiratory Society ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624.
- Sclauser Pessoa IM, Franco Parreira V, Fregonezi GA, et al. Reference values for maximal inspiratory pressure: a systematic review. Can Respir J. 2014;21(1):43–50.
- Bissett B, Gosselink R, van Haren FMP. Respiratory muscle rehabilitation in patients with prolonged mechanical ventilation: a targeted approach. Crit Care. 2020;24(1):103.
- Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117.
- Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1384–1387.
- Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health. 2013;58(6):949–953.
- Finnsback C, Mannerkorpi K. Spinal and thoracic mobility-age-related reference values for healthy men and women. Nordisk Fysioterapi. 2005;9:136–143.
- Olsén MF, Lindstrand H, Broberg JL, et al. Measuring chest expansion; a study comparing two different instructions. Adv Physiother. 2011;13(3):128–132.
- Ragnarsdottir M, Kristinsdottir EK. Breathing movements and breathing patterns among healthy men and women 20-69 years of age. Reference values. Respiration. 2006;73(1):48–54.
- Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991.
- McConnell A. Respiratory muscle training: theory and practice. London: Churchill Livingstone Elsevier; 2013.
- Moll JM, Wright V. An objective clinical study of chest expansion. Ann Rheum Dis. 1972;31(1):1–8.
- Cortes-Telles A, Lopez-Romero S, Figueroa-Hurtado E, et al. Pulmonary function, and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir Physiol Neurobiol. 2021;288:103644.
- Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology, and management strategies. Clin Med (Lond). 2021;21(1):e63–e67.
- Björnson M, Caidahl K, Loewenstein D, et al. Jämförelse av demografi och fysisk funktion hos sjukhusvårdade och hemmavårdade patienter med postakut covid-19 syndrom - en observationsstudie. State of the Art Covid-19, Svenska Läkarsällskapet; 2021.

